Epigenetic Explorations of Neurological Disorders, the Identification Methods, and Therapeutic Avenues
- PMID: 39519209
- PMCID: PMC11546397
- DOI: 10.3390/ijms252111658
Epigenetic Explorations of Neurological Disorders, the Identification Methods, and Therapeutic Avenues
Abstract
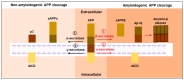
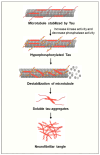
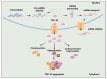
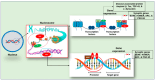
Neurodegenerative disorders are major health concerns globally, especially in aging societies. The exploration of brain epigenomes, which consist of multiple forms of DNA methylation and covalent histone modifications, offers new and unanticipated perspective into the mechanisms of aging and neurodegenerative diseases. Initially, chromatin defects in the brain were thought to be static abnormalities from early development associated with rare genetic syndromes. However, it is now evident that mutations and the dysregulation of the epigenetic machinery extend across a broader spectrum, encompassing adult-onset neurodegenerative diseases. Hence, it is crucial to develop methodologies that can enhance epigenetic research. Several approaches have been created to investigate alterations in epigenetics on a spectrum of scales-ranging from low to high-with a particular focus on detecting DNA methylation and histone modifications. This article explores the burgeoning realm of neuroepigenetics, emphasizing its role in enhancing our mechanistic comprehension of neurodegenerative disorders and elucidating the predominant techniques employed for detecting modifications in the epigenome. Additionally, we ponder the potential influence of these advancements on shaping future therapeutic approaches.
Keywords: Alzheimer’s disease; DNA methylation; Parkinsons’s disease; amyotrophic lateral sclerosis; histone acetylation; neurodegeneration; neuroepigenetics.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Epigenetic mechanisms in neurological disease.Nat Med. 2012 Aug;18(8):1194-204. doi: 10.1038/nm.2828. Nat Med. 2012. PMID: 22869198 Free PMC article. Review.
-
Histone Methylation Regulation in Neurodegenerative Disorders.Int J Mol Sci. 2021 Apr 28;22(9):4654. doi: 10.3390/ijms22094654. Int J Mol Sci. 2021. PMID: 33925016 Free PMC article. Review.
-
Epigenomics in stress tolerance of plants under the climate change.Mol Biol Rep. 2023 Jul;50(7):6201-6216. doi: 10.1007/s11033-023-08539-6. Epub 2023 Jun 9. Mol Biol Rep. 2023. PMID: 37294468 Review.
-
Laboratory methods to decipher epigenetic signatures: a comparative review.Cell Mol Biol Lett. 2021 Nov 11;26(1):46. doi: 10.1186/s11658-021-00290-9. Cell Mol Biol Lett. 2021. PMID: 34763654 Free PMC article. Review.
-
The tale of histone modifications and its role in multiple sclerosis.Hum Genomics. 2018 Jun 22;12(1):31. doi: 10.1186/s40246-018-0163-5. Hum Genomics. 2018. PMID: 29933755 Free PMC article. Review.
Cited by
-
Decoding Neurodegeneration: A Review of Molecular Mechanisms and Therapeutic Advances in Alzheimer's, Parkinson's, and ALS.Int J Mol Sci. 2024 Nov 24;25(23):12613. doi: 10.3390/ijms252312613. Int J Mol Sci. 2024. PMID: 39684324 Free PMC article. Review.
References
-
- Nichols E., Steinmetz J.D., Vollset S.E., Fukutaki K., Chalek J., Abd-Allah F., Abdoli A., Abualhasan A., Abu-Gharbieh E., Akram T.T. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105–e125. doi: 10.1016/S2468-2667(21)00249-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

