Chitin Translocation Is Functionally Coupled with Synthesis in Chitin Synthase
- PMID: 39519219
- PMCID: PMC11546553
- DOI: 10.3390/ijms252111667
Chitin Translocation Is Functionally Coupled with Synthesis in Chitin Synthase
Abstract
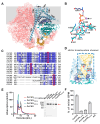
Chitin, an extracellular polysaccharide, is synthesized by membrane-embedded chitin synthase (CHS) utilizing intracellular substrates. The mechanism of the translocation of synthesized chitin across the membrane to extracellular locations remains unresolved. We prove that the chitin synthase from Phytophthora sojae (PsCHS) is a processive glycosyltransferase, which can rapidly produce and tightly bind with the highly polymerized chitin. We further demonstrate that PsCHS is a bifunctional enzyme, which is necessary and sufficient to translocate the synthesized chitin. PsCHS was purified and then reconstituted into proteoliposomes (PLs). The nascent chitin is generated and protected from chitinase degradation unless detergent solubilizes the PLs, showing that PsCHS translocates the newly produced chitin into the lumen of the PLs. We also attempted to resolve the PsCHS structure of the synthesized chitin-bound state, although it was not successful; the obtained high-resolution structure of the UDP/Mn2+-bound state could still assist in describing the characterization of the PsCHS's transmembrane channel. Consistently, we demonstrate that PsCHS is indispensable and capable of translocating chitin in a process that is tightly coupled to chitin synthesis.
Keywords: chitin synthase; glycosyltransferase; membrane translocation; processive.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Structural basis for directional chitin biosynthesis.Nature. 2022 Oct;610(7931):402-408. doi: 10.1038/s41586-022-05244-5. Epub 2022 Sep 21. Nature. 2022. PMID: 36131020 Free PMC article.
-
The hyaluronan synthase catalyzes the synthesis and membrane translocation of hyaluronan.J Mol Biol. 2012 Apr 20;418(1-2):21-31. doi: 10.1016/j.jmb.2012.01.053. Epub 2012 Feb 13. J Mol Biol. 2012. PMID: 22343360
-
Independent regulation of chitin synthase and chitinase activity in Candida albicans and Saccharomyces cerevisiae.Microbiology (Reading). 2004 Apr;150(Pt 4):921-928. doi: 10.1099/mic.0.26661-0. Microbiology (Reading). 2004. PMID: 15073301
-
Chitin Synthesis and Degradation in Fungi: Biology and Enzymes.Adv Exp Med Biol. 2019;1142:153-167. doi: 10.1007/978-981-13-7318-3_8. Adv Exp Med Biol. 2019. PMID: 31102246 Review.
-
Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases.J Exp Biol. 2003 Dec;206(Pt 24):4393-412. doi: 10.1242/jeb.00709. J Exp Biol. 2003. PMID: 14610026 Review.
References
-
- Brown H.E., Esher S.K., Alspaugh J.A. Chitin: A “Hidden Figure” in the fungal cell wall. Curr. Top. Microbiol. Immunol. 2020;425:83–111. - PubMed
MeSH terms
Substances
Grants and funding
- 2023YFE0125400/National Key R&D Program of China
- 42376136/National Natural Science Foundation of China
- SKLSGB-ORP202105/the Opening fund of State Key Laboratory of Silkworm Genome Biology
- ts20230204/the Instrument Improvement Funds of Shandong University Public Technology Platform
- DKXZZ202205/Research on Simulation Technology and Device of Key Processes of Typical Marine Ecological Disasters in the Pre-Research Project of Major Scientific Facilities in Shandong Province
LinkOut - more resources
Full Text Sources

