SMI-Capsular Fibrosis and Biofilm Dynamics: Molecular Mechanisms, Clinical Implications, and Antimicrobial Approaches
- PMID: 39519227
- PMCID: PMC11546664
- DOI: 10.3390/ijms252111675
SMI-Capsular Fibrosis and Biofilm Dynamics: Molecular Mechanisms, Clinical Implications, and Antimicrobial Approaches
Abstract
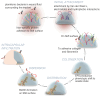
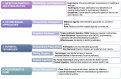
Silicone mammary implants (SMIs) frequently result in capsular fibrosis, which is marked by the overproduction of fibrous tissue surrounding the implant. This review provides a detailed examination of the molecular and immunological mechanisms driving capsular fibrosis, focusing on the role of foreign body responses (FBRs) and microbial biofilm formation. We investigate how microbial adhesion to implant surfaces and biofilm development contribute to persistent inflammation and fibrotic responses. The review critically evaluates antimicrobial strategies, including preoperative antiseptic protocols and antimicrobial-impregnated materials, designed to mitigate infection and biofilm-related complications. Additionally, advancements in material science, such as surface modifications and antibiotic-impregnated meshes, are discussed for their potential to reduce capsular fibrosis and prevent contracture of the capsule. By integrating molecular insights with clinical applications, this review aims to elucidate the current understanding of SMI-related fibrotic responses and highlight knowledge gaps. The synthesis of these findings aims to guide future research directions of improved antimicrobial interventions and implant materials, ultimately advancing the management of capsular fibrosis and enhancing patient outcomes.
Keywords: antimicrobial-impregnated materials; biofilm formation; capsular fibrosis; fibrotic response; foreign body response (FBR); implant surface modification; silicone mammary implants (SMIs).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Safran T., Nepon H., Chu C.K., Winocour S., Murphy A.M., Davison P.G., Dionisopolos T., Vorstenbosch J. Healing, inflammation, and fibrosis: Current concepts in capsular contracture: Pathophysiology, prevention, and management. Semin. Plast. Surg. 2021;35:189–202. doi: 10.1055/s-0041-1731793. - DOI - PMC - PubMed
-
- Kadirvelu L., Sri Sivaramalingam S., Jothivel D., Dharshika Chithiraiselvan D., Karaiyagowder Govindarajan D., Kandaswamy K. A review on antimicrobial strategies in mitigating biofilm-associated infections on medical implants. Curr. Res. Microb. Sci. 2024;6:100231. doi: 10.1016/j.crmicr.2024.100231. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

