Beyond Recycling Antibodies: Crovalimab's Molecular Design Enables Four-Weekly Subcutaneous Injections for PNH Treatment
- PMID: 39519232
- PMCID: PMC11546984
- DOI: 10.3390/ijms252111679
Beyond Recycling Antibodies: Crovalimab's Molecular Design Enables Four-Weekly Subcutaneous Injections for PNH Treatment
Abstract
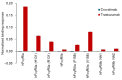
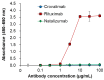
The advent of recycling antibodies, leveraging pH-dependent antigen binding and optimized FcRn interaction, has advanced the field of antibody therapies, enabling extended durability and reduced dosages. Eculizumab (Soliris®) demonstrated the efficacy of C5 inhibitors for paroxysmal nocturnal hemoglobinuria (PNH), while its derivative, ravulizumab (Ultomiris®), recognized as a recycling antibody, extended the dosing intervals. However, limitations including intravenous administration and inefficacy in patients with the R885H single-nucleotide polymorphism (SNP) in C5 could necessitate alternative solutions. Crovalimab (PiaSky®), a next-generation recycling antibody, overcomes these challenges with innovative charge engineering, achieving the enhanced cellular uptake of C5-crovalimab complexes and targeting a unique C5 epitope, allowing for efficacy regardless of the R885H SNP. This study highlights crovalimab's distinctive molecular features, showing its eliminated binding to Fcγ receptors and C1q, alongside its optimized antigen binding characteristics. The impact of charge engineering was reconfirmed in mice, demonstrating faster C5 clearance than recycling antibodies. Notably, in the maintenance dosing regimen, crovalimab neutralizes approximately seven C5 molecules per antibody on average. Furthermore, its design also reduces the viscosity to facilitate high-concentration formulations suitable for subcutaneous delivery. Consequently, crovalimab offers a four-weekly subcutaneous injection regimen for PNH, marking a substantial improvement in treatment convenience and potentially transforming patients' quality of life.
Keywords: anti-C5 antibody; charge engineering; crovalimab (PiaSky®); eculizumab; pH-dependent antigen binding; paroxysmal nocturnal hemoglobinuria; ravulizumab; recycling antibody; subcutaneous injection; viscosity.
Conflict of interest statement
Z.S., K.H., M.M., T.F. and M.S.-K. were employed by Chugai Pharmabody Research Pte., Ltd. at the time of this study. A.H. was employed by Chugai Pharmaceutical Co., Ltd. at the time of this study. Z.S., K.H., M.M., T.F., M.S.-K. and Y.T. are currently employed by Chugai Pharmaceutical Co., Ltd. S.W.G. is currently employed by Chugai Pharmabody Research Pte., Ltd. A.G. reports research funding from Eisai Co., Ltd., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., Chugai Pharmaceutical Co., Ltd., MSD K.K., Otsuka Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Bayer Yakuhin, Ltd., Daiichi Sankyo Co., Ltd., and Nihon Pharmaceutical Co., Ltd.; consulting fees from PharmaEssentia Japan K.K., Chugai Pharmaceutical Co., Ltd., Alexion Pharmaceuticals, Inc., and Asahi Kasei Pharma Corp.; honoraria from Novartis Pharma K.K., Alexion Pharmaceuticals, Inc., Eisai Co., Ltd., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., Chugai Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Nihon Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Janssen Pharmaceutical K.K., Pfizer Japan Inc., Sanofi K.K., Asahi Kasei Pharma Corp., and PharmaEssentia Japan K.K.; and participation on a Data Safety Monitoring or Advisory Board for PharmaEssentia Japan K.K., Chugai Pharmaceutical Co., Ltd., Alexion Pharmaceuticals, Inc., and Asahi Kasei Pharma Corp. N.O. reports research funding from Alexion Pharmaceuticals, Inc.; honorarium/lecture fees from Alexion Pharmaceuticals, Inc., Novartis Pharma K.K., Swedish Orphan Biovitrum AB (publ), Chugai Pharmaceutical Co., Ltd., F. Hoffmann-La Roche, Ltd., and Kyowa Kirin Co., Ltd. Y.U. reports an advisory role for Alexion Pharmaceuticals, Inc., Asahi Kasei Pharma Corp., Chugai Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Novartis Pharma K.K., and Sanofi K.K.; honoraria/lecture fees from Alexion Pharmaceuticals, Inc., Chugai Pharmaceutical Co., Ltd., Incyte Biosciences Japan G.K., Janssen Pharmaceutical K.K., Kaken Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Sanofi K.K., and Swedish Orphan Biovitrum AB (publ); and research funding from Chugai Pharmaceutical Co., Ltd. All authors received research support in the form of third-party medical writing assistance for this manuscript, provided by Akshaya Srinivasan, PhD, CMPP, of Nucleus Global, an Inizio company, from Chugai Pharmaceutical Co., Ltd. The authors report the filing of the following patent applications relevant to crovalimab. Z.S. is the inventor on the patent applications “Anti-C5 antibodies and methods of use” (WO/2016/098356), “Anti-C5 antibodies and methods of use” (WO/2017/217524), and “Anti-C5 antibodies and methods of use” (WO/2017/104779). Z.S., K.H., T.F., and Y.T. are listed as inventors on the patent application “A pharmaceutical composition for use in the treatment or prevention of a C5-related disease and a method for treating or preventing a C5-related disease” (WO/2018/143266). All authors declare that this research was conducted in the absence of any other commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Richards S.J., Painter D., Dickinson A.J., Griffin M., Munir T., Arnold L., Payne D., Pike A., Muus P., Hill A., et al. The incidence and prevalence of patients with paroxysmal nocturnal haemoglobinuria and aplastic anaemia PNH syndrome: A retrospective analysis of the UK’s population-based haematological malignancy research network 2004–2018. Eur. J. Haematol. 2021;107:211–218. doi: 10.1111/ejh.13640. - DOI - PubMed
-
- Jalbert J.J., Chaudhari U., Zhang H., Weyne J., Shammo J.M. Epidemiology of PNH and real-world treatment patterns following an incident PNH diagnosis in the US. Blood. 2019;134:3407. doi: 10.1182/blood-2019-125867. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

