Amino Acid Compound 2 (AAC2) Treatment Counteracts Insulin-Induced Synaptic Gene Expression and Seizure-Related Mortality in a Mouse Model of Alzheimer's Disease
- PMID: 39519239
- PMCID: PMC11546384
- DOI: 10.3390/ijms252111689
Amino Acid Compound 2 (AAC2) Treatment Counteracts Insulin-Induced Synaptic Gene Expression and Seizure-Related Mortality in a Mouse Model of Alzheimer's Disease
Abstract
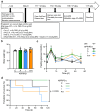
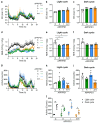
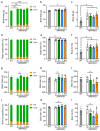
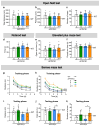
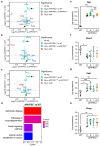
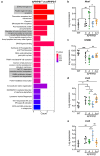
Diabetes is a major risk factor for Alzheimer's disease (AD). Amino acid compound 2 (AAC2) improves glycemic and cognitive functions in diabetic mouse models through mechanisms distinct from insulin. Our goal was to compare the effects of AAC2, insulin, and their nanofiber-forming combination on early asymptomatic AD pathogenesis in APP/PS1 mice. Insulin, but not AAC2 or the combination treatment (administered intraperitoneally every 48 h for 120 days), increased seizure-related mortality, altered the brain fat-to-lean mass ratio, and improved specific cognitive functions in APP/PS1 mice. NanoString and pathway analysis of cerebral gene expression revealed dysregulated synaptic mechanisms, with upregulation of Bdnf and downregulation of Slc1a6 in insulin-treated mice, correlating with insulin-induced seizures. In contrast, AAC2 promoted the expression of Syn2 and Syp synaptic genes, preserved brain composition, and improved survival. The combination of AAC2 and insulin counteracted free insulin's effects. None of the treatments influenced canonical amyloidogenic pathways. This study highlights AAC2's potential in regulating synaptic gene expression in AD and insulin-induced contexts related to seizure activity.
Keywords: Alzheimer’s disease; cognitive; dementia; diabetes; dipeptides; epilepsy; insulin; nanofibers; nanomaterials; seizures; synapses.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Mitigation of synaptic and memory impairments via F-actin stabilization in Alzheimer's disease.Alzheimers Res Ther. 2024 Sep 7;16(1):200. doi: 10.1186/s13195-024-01558-w. Alzheimers Res Ther. 2024. PMID: 39244567 Free PMC article.
-
Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer's disease.Neuropharmacology. 2014 Jan;76 Pt A:57-67. doi: 10.1016/j.neuropharm.2013.08.005. Epub 2013 Aug 21. Neuropharmacology. 2014. PMID: 23973293
-
Novel Phenylethanoid Glycosides Improve Hippocampal Synaptic Plasticity via the Cyclic Adenosine Monophosphate-CREB-Brain-Derived Neurotrophic Growth Factor Pathway in APP/PS1 Transgenic Mice.Gerontology. 2023;69(9):1065-1075. doi: 10.1159/000531194. Epub 2023 Jun 7. Gerontology. 2023. PMID: 37285833 Free PMC article.
-
L-3-n-butylphthalide Rescues Hippocampal Synaptic Failure and Attenuates Neuropathology in Aged APP/PS1 Mouse Model of Alzheimer's Disease.CNS Neurosci Ther. 2016 Dec;22(12):979-987. doi: 10.1111/cns.12594. Epub 2016 Jul 20. CNS Neurosci Ther. 2016. PMID: 27439966 Free PMC article.
-
Amino Acid Nanofibers Improve Glycemia and Confer Cognitive Therapeutic Efficacy to Bound Insulin.Pharmaceutics. 2021 Dec 29;14(1):81. doi: 10.3390/pharmaceutics14010081. Pharmaceutics. 2021. PMID: 35056977 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

