Molecular and Cellular Mechanisms Involved in the Pathophysiology of Retinal Vascular Disease-Interplay Between Inflammation and Oxidative Stress
- PMID: 39519401
- PMCID: PMC11546760
- DOI: 10.3390/ijms252111850
Molecular and Cellular Mechanisms Involved in the Pathophysiology of Retinal Vascular Disease-Interplay Between Inflammation and Oxidative Stress
Abstract
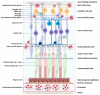
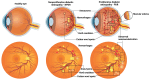
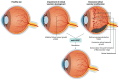
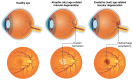
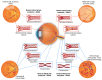
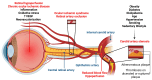
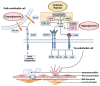
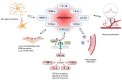
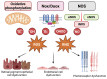
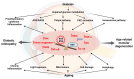
Retinal vascular diseases encompass several retinal disorders, including diabetic retinopathy, retinopathy of prematurity, age-related macular degeneration, and retinal vascular occlusion; these disorders are classified as similar groups of disorders due to impaired retinal vascularization. The aim of this review is to address the main signaling pathways involved in the pathogenesis of retinal vascular diseases and to identify crucial molecules and the importance of their interactions. Vascular endothelial growth factor (VEGF) is recognized as a crucial and central molecule in abnormal neovascularization and a key phenomenon in retinal vascular occlusion; thus, anti-VEGF therapy is now the most successful form of treatment for these disorders. Interaction between angiopoietin 2 and the Tie2 receptor results in aberrant Tie2 signaling, resulting in loss of pericytes, neovascularization, and inflammation. Notch signaling and hypoxia-inducible factors in ischemic conditions induce pathological neovascularization and disruption of the blood-retina barrier. An increase in the pro-inflammatory cytokines-TNF-α, IL-1β, and IL-6-and activation of microglia create a persistent inflammatory milieu that promotes breakage of the blood-retinal barrier and neovascularization. Toll-like receptor signaling and nuclear factor-kappa B are important factors in the dysregulation of the immune response in retinal vascular diseases. Increased production of reactive oxygen species and oxidative damage follow inflammation and together create a vicious cycle because each factor amplifies the other. Understanding the complex interplay among various signaling pathways, signaling cascades, and molecules enables the development of new and more successful therapeutic options.
Keywords: angiogenesis; cellular signaling; retinal vascular diseases; vascular endothelial growth factor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
[Aging and retinal vascular diseases].Nippon Ganka Gakkai Zasshi. 2007 Mar;111(3):207-30; discussion 231. Nippon Ganka Gakkai Zasshi. 2007. PMID: 17402563 Review. Japanese.
-
Molecular pathogenesis of retinal and choroidal vascular diseases.Prog Retin Eye Res. 2015 Nov;49:67-81. doi: 10.1016/j.preteyeres.2015.06.002. Epub 2015 Jun 23. Prog Retin Eye Res. 2015. PMID: 26113211 Free PMC article. Review.
-
Pericyte-Endothelial Interactions in the Retinal Microvasculature.Int J Mol Sci. 2020 Oct 8;21(19):7413. doi: 10.3390/ijms21197413. Int J Mol Sci. 2020. PMID: 33049983 Free PMC article. Review.
-
Vascular permeability in retinopathy is regulated by VEGFR2 Y949 signaling to VE-cadherin.Elife. 2020 Apr 21;9:e54056. doi: 10.7554/eLife.54056. Elife. 2020. PMID: 32312382 Free PMC article.
-
VEGFR1 signaling in retinal angiogenesis and microinflammation.Prog Retin Eye Res. 2021 Sep;84:100954. doi: 10.1016/j.preteyeres.2021.100954. Epub 2021 Feb 25. Prog Retin Eye Res. 2021. PMID: 33640465 Free PMC article. Review.
Cited by
-
Exploring Endothelial Cell Dysfunction's Impact on the Brain-Retina Microenvironment Connection: Molecular Mechanisms and Implications.Mol Neurobiol. 2025 Jun;62(6):7484-7505. doi: 10.1007/s12035-025-04714-x. Epub 2025 Feb 4. Mol Neurobiol. 2025. PMID: 39904964 Review.
-
Pinosylvin: A Multifunctional Stilbenoid with Antimicrobial, Antioxidant, and Anti-Inflammatory Potential.Curr Issues Mol Biol. 2025 Mar 18;47(3):204. doi: 10.3390/cimb47030204. Curr Issues Mol Biol. 2025. PMID: 40136458 Free PMC article. Review.
-
Ceruloplasmin and Ferritin Changes in Ocular Fluids from Patients with Vitreoretinal Diseases: Relation with Neuroinflammation and Drusen Formation.Int J Mol Sci. 2025 Jun 30;26(13):6307. doi: 10.3390/ijms26136307. Int J Mol Sci. 2025. PMID: 40650085 Free PMC article.
-
Recent advances in engineered exosome-based therapies for ocular vascular disease.J Nanobiotechnology. 2025 Jul 19;23(1):526. doi: 10.1186/s12951-025-03589-3. J Nanobiotechnology. 2025. PMID: 40684186 Free PMC article. Review.
-
hsa_circ_0099682 exacerbates the development of diabetic retinopathy by promoting pathological neoangiogenesis via the miR-125b-5p/HNRNPU axis.J Mol Histol. 2025 Aug 9;56(4):264. doi: 10.1007/s10735-025-10535-y. J Mol Histol. 2025. PMID: 40782251
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

