Cytoprotective Effects of Antioxidant Peptides from Red Californian Worm (Eisenia foetida) Hydrolysate on Differentiated Caco-2 Cells
- PMID: 39519487
- PMCID: PMC11547318
- DOI: 10.3390/nu16213654
Cytoprotective Effects of Antioxidant Peptides from Red Californian Worm (Eisenia foetida) Hydrolysate on Differentiated Caco-2 Cells
Abstract
Background/objectives: When prooxidants outweigh antioxidants, oxidative stress can occur, causing an accumulation of reactive oxygen species (ROS). This process can lead to cellular damage and plays a role in the development of numerous health conditions. This study aimed to investigate the cytoprotective effects on differentiated Caco-2 cells of hydrolysates derived from the red Californian worm (WH) and their fractions, identify the peptides responsible for this effect, and elucidate the mechanisms involved.
Methods: The WH was obtained through hydrolysis with Alcalase 2.4 L and subsequently fractionated to two fractions (F > 3 kDa and F < 3 kDa) using a ceramic membrane with a molecular weight cutoff of 3 kDa. The peptides found in the F < 3 kDa fraction, demonstrating the highest cytoprotective activity, were then sequenced via liquid chromatography-mass spectrometry analysis (LC-MS/MS), and molecular docking was conducted to elucidate the underlying antioxidant mechanisms.
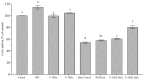
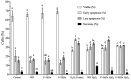
Results: The hydrolysate of Eisenia foetida and its F < 3 kDa fraction exhibited no cytotoxicity, protected the cells from H2O2-induced oxidative stress (50% increase viability), preserved cell viability by restoring their redox status (ROS: 20% decrease, and glutathione (GSH): recovered to basal control levels) and cell cycle distribution, and decreased apoptosis (16%). Twenty-eight peptides were identified, with five showing antioxidant activity through stable interactions with myeloperoxidase (MPO) and Kelch-like ECH-associated protein 1 (Keap-1), KPEDWDDR being the peptide that presented the highest affinity with both molecules (-7.9 and -8.8 kCal/mol, respectively).
Conclusions: These results highlight the WH as a potential source of bioactive peptides for the management of oxidative stress.
Keywords: antioxidant peptide; cytoprotective effect; molecular docking; oxidative stress; red Californian worm (Eisenia foetida).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Novel anti-oxidative peptides from equine hemoplasma protein hydrolysates: Purification, identification and protective effects on Caco-2 cells.Food Res Int. 2025 Mar;204:115943. doi: 10.1016/j.foodres.2025.115943. Epub 2025 Feb 5. Food Res Int. 2025. PMID: 39986787
-
Purification and Characterization of Antioxidant Peptides from Alcalase-Hydrolyzed Soybean ( Glycine max L.) Hydrolysate and Their Cytoprotective Effects in Human Intestinal Caco-2 Cells.J Agric Food Chem. 2019 May 22;67(20):5772-5781. doi: 10.1021/acs.jafc.9b01235. Epub 2019 May 9. J Agric Food Chem. 2019. PMID: 31046268
-
Antioxidant activity and protective effects of Alcalase-hydrolyzed soybean hydrolysate in human intestinal epithelial Caco-2 cells.Food Res Int. 2018 Sep;111:256-264. doi: 10.1016/j.foodres.2018.05.046. Epub 2018 May 21. Food Res Int. 2018. PMID: 30007684
-
Antioxidant Peptides from the Protein Hydrolysate of Monkfish (Lophius litulon) Muscle: Purification, Identification, and Cytoprotective Function on HepG2 Cells Damage by H2O2.Mar Drugs. 2020 Mar 10;18(3):153. doi: 10.3390/md18030153. Mar Drugs. 2020. PMID: 32164197 Free PMC article.
-
In-vitro antioxidant capacity and cytoprotective/cytotoxic effects upon Caco-2 cells of red tilapia (Oreochromis spp.) viscera hydrolysates.Food Res Int. 2019 Jun;120:52-61. doi: 10.1016/j.foodres.2019.02.029. Epub 2019 Feb 19. Food Res Int. 2019. PMID: 31000267
References
-
- Onel L., Morales H.G., Nayade D., Roche P., Rosa D., Flores Sánchez M. Enzimas generadoras de especies reactivas del oxígeno: Mieloperoxidasa. Rev. Cuba. Investig. Biomed. 1998;17:190–197.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

