S-Adenosylmethionine (SAMe) for Liver Health: A Systematic Review
- PMID: 39519500
- PMCID: PMC11547595
- DOI: 10.3390/nu16213668
S-Adenosylmethionine (SAMe) for Liver Health: A Systematic Review
Abstract
Background/objectives: S-adenosylmethionine (SAMe) is a natural compound implicated in the treatment of liver dysfunction. In this systematic review, our objective was to determine the efficacy, safety, and optimal dose of SAMe in liver diseases.
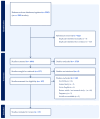
Methods: Using the PRISMA methodology, we searched PubMed, CINAHL, and Web of Science using key MeSH search terms. For title/abstract screening, full-text review, and data extraction, two independent researchers reviewed articles, and a third researcher resolved conflicts. Data extraction also included a quality assessment of included articles.
Results: Of the 1881 non-duplicated studies, 15 articles focusing on SAMe use in the liver were included. All included studies (n = 15) scored a 4 or 5 out of 5 points on the quality assessment, which indicated high study quality. Overall, SAMe was effective in improving liver-related parameters with few adverse events, which were primarily mild, transient gastrointestinal complaints.
Conclusions: The most common doses were SAMe 1000 mg or 1200 mg per day with or without another treatment or natural supplement. Future studies are needed to assess long-term efficacy and safety data of SAMe and the optimal route of administration in liver diseases.
Keywords: S-adenosylmethionine; SAMe; liver; nutraceutical.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
S-Adenosylmethionine (SAMe) for Central Nervous System Health: A Systematic Review.Nutrients. 2024 Sep 18;16(18):3148. doi: 10.3390/nu16183148. Nutrients. 2024. PMID: 39339750 Free PMC article.
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
-
Chronic liver diseases and the potential use of S-adenosyl-L-methionine as a hepatoprotector.Eur J Gastroenterol Hepatol. 2018 Aug;30(8):893-900. doi: 10.1097/MEG.0000000000001141. Eur J Gastroenterol Hepatol. 2018. PMID: 29683981 Review.
-
Efficacy of the dietary supplement S-adenosyl-L-methionine.Ann Pharmacother. 2001 Nov;35(11):1414-25. doi: 10.1345/aph.1Z443. Ann Pharmacother. 2001. PMID: 11724095
Cited by
-
KASL clinical practice guidelines for the management of metabolic dysfunction-associated steatotic liver disease 2025.Clin Mol Hepatol. 2025 Feb;31(Suppl):S1-S31. doi: 10.3350/cmh.2025.0045. Epub 2025 Feb 19. Clin Mol Hepatol. 2025. PMID: 39967303 Free PMC article. No abstract available.
References
-
- Centers for Disease Control and Prevention Chronic Liver Disease and Cirrhosis. [(accessed on 10 September 2024)]; Available online: https://www.cdc.gov/nchs/fastats/liver-disease.htm.
-
- Xu J.Q. Trends in Liver Cancer Mortality Among Adults Aged 25 and over in the United States, 2000–2016. National Center for Health Statistics; Hyattsville, MD, USA: 2018. NCHS Data Brief, no 314.
-
- Riazi K., Azhari H., Charette J.H., Underwood F.E., King J.A., Afshar E.E., Swain M.G., Congly S.E., Kaplan G.G., Shaheen A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical