Amelioration of Cancer Cachexia by Dalbergia odorifera Extract Through AKT Signaling Pathway Regulation
- PMID: 39519503
- PMCID: PMC11547832
- DOI: 10.3390/nu16213671
Amelioration of Cancer Cachexia by Dalbergia odorifera Extract Through AKT Signaling Pathway Regulation
Abstract
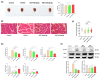
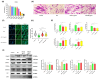
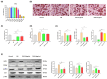
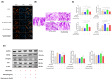
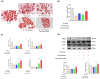
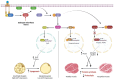
Background/Objectives: Cancer cachexia is a multifactorial syndrome characterized by the progressive loss of skeletal muscle mass and adipose tissue. Dalbergia odorifer is widely used in traditional medicine in Korea and China to treat various diseases. However, its exact role and underlying mechanism in regulating cancer cachexia have not been elucidated yet. This research was conducted to investigate the effect of D. odorifer extract (DOE) in preventing the development of cancer-induced cachexia symptoms and figure out the relevant mechanisms. Methods: A cancer cachexia model was established in Balb/c mice using the CT26 colon carcinoma cell line. To evaluate the anti-cachexia effect of Dalbergia odorifer extract (DOE), CT26-bearing mice were orally administered with DOE at concentrations of 50 and 100 mg/kg BW for 14 days. C2C12 myotubes and 3T3L1 adipocytes were treated with 80% CT26 conditioned medium, DOE, and wortmannin, a particular AKT inhibitor to determine the influence of DOE in the AKT signaling pathway. Mice body weight, food intake, myofiber cross-sectional area, adipocyte size, myotube diameter, lipid accumulation, and relevant gene expression were analyzed. Results: The oral administration of DOE at doses of 50 and 100 mg/kg body weight to CT26 tumor-bearing mice resulted in a significant reduction in body weight loss, an increase in food intake, and a decrease in serum glycerol levels. Furthermore, DOE treatment led to an increase in muscle mass, larger muscle fiber diameter, and elevated expression levels of MyH2 and Igf1, while simultaneously reducing the expression of Atrogin1 and MuRF1. DOE also attenuated adipose tissue wasting, as evidenced by increased epididymal fat mass, enlarged adipocyte size, and upregulated Pparγ expression, alongside a reduction in Ucp1 and IL6 levels. In cachectic C2C12 myotubes and 3T3-L1 adipocytes induced by the CT26 conditioned medium, DOE significantly inhibited muscle wasting and lipolysis by activating the AKT signaling pathway. The treatment of wortmannin, a specific AKT inhibitor, effectively neutralized DOE's impact on the AKT pathway, myotube diameter, and lipid accumulation. Conclusions: DOE ameliorates cancer cachexia through the expression of genes involved in protein synthesis and lipogenesis, while suppressing those related to protein degradation, suggesting its potential as a plant-derived therapeutic agent in combating cancer cachexia.
Keywords: AKT; Dalbergia odorifer extract; adipose wasting; cancer cachexia; muscle atrophy.
Conflict of interest statement
Author Eulyong Park was employed by the company Easthill Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Cryptotanshinone prevents muscle wasting in CT26-induced cancer cachexia through inhibiting STAT3 signaling pathway.J Ethnopharmacol. 2020 Oct 5;260:113066. doi: 10.1016/j.jep.2020.113066. Epub 2020 Jun 4. J Ethnopharmacol. 2020. PMID: 32505837
-
Matrine improves skeletal muscle atrophy by inhibiting E3 ubiquitin ligases and activating the Akt/mTOR/FoxO3α signaling pathway in C2C12 myotubes and mice.Oncol Rep. 2019 Aug;42(2):479-494. doi: 10.3892/or.2019.7205. Epub 2019 Jun 19. Oncol Rep. 2019. PMID: 31233199 Free PMC article.
-
Valproic acid attenuates skeletal muscle wasting by inhibiting C/EBPβ-regulated atrogin1 expression in cancer cachexia.Am J Physiol Cell Physiol. 2016 Jul 1;311(1):C101-15. doi: 10.1152/ajpcell.00344.2015. Epub 2016 Apr 27. Am J Physiol Cell Physiol. 2016. PMID: 27122162
-
Selumetinib Attenuates Skeletal Muscle Wasting in Murine Cachexia Model through ERK Inhibition and AKT Activation.Mol Cancer Ther. 2017 Feb;16(2):334-343. doi: 10.1158/1535-7163.MCT-16-0324. Epub 2016 Sep 6. Mol Cancer Ther. 2017. PMID: 27599525
-
New insights into adipose tissue atrophy in cancer cachexia.Proc Nutr Soc. 2009 Nov;68(4):385-92. doi: 10.1017/S0029665109990267. Epub 2009 Sep 1. Proc Nutr Soc. 2009. PMID: 19719894 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

