The Efficacy of Omega-3 Fatty Acids as the Monotherapy for Depression: A Randomized, Double-Blind, Placebo-Controlled Pilot Study
- PMID: 39519521
- PMCID: PMC11547719
- DOI: 10.3390/nu16213688
The Efficacy of Omega-3 Fatty Acids as the Monotherapy for Depression: A Randomized, Double-Blind, Placebo-Controlled Pilot Study
Abstract
Objective: Omega-3 polyunsaturated fatty acids (n-3 PUFAs) have demonstrated protective effects in major depressive disorder (MDD) patients receiving antidepressant treatment. However, there have been a few double-blind randomized controlled trials focused on n-3 PUFAs as monotherapy in MDD, and the outcomes have been mixed. This study aimed to assess the clinical effects of n-3 PUFAs monotherapy in patients with MDD.
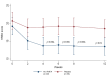
Methods: A total of 60 patients with MDD participated in this 12-week double-blind randomized controlled trial. They were randomized to either the n-3 PUFAs group (n = 30; 3.2 g of eicosapentaenoic acid, EPA and docosahexaenoic acid, DHA per day) or the placebo group (n = 30; 3.2 g of soybean oil per day). The severity of depression was evaluated using the Hamilton Rating Scale for Depression (HRSD).
Results: The n-3 PUFAs group had a significantly lower HRSD score compared with the placebo group at week 4 (p = 0.004), week 6 (p = 0.006), week 8 (p = 0.004), and week 12 (p = 0.01). The n-3 PUFAs group showed slightly higher rates for both remission (26.7% vs. 10%, p = 0.095) and response (23.3% vs. 6.7%, p = 0.145) compared with the placebo group at week 12, but these differences did not reach statistical significance.
Conclusions: These findings suggested that monotherapy of n-3 PUFAs could improve depression and potentially serve as an alternative option for MDD patients.
Keywords: RCT; depression; monotherapy; n-3 PUFAs.
Conflict of interest statement
The authors declare that they have no financial or other relationships that might lead to a conflict of interest.
Figures

References
-
- Trivedi M.H., Rush A.J., Wisniewski S.R., Nierenberg A.A., Warden D., Ritz L., Norquist G., Howland R.H., Lebowitz B., McGrath P.J., et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am. J. Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NSTC 109-2320-B-038-057-MY3, 110-2321-B-006-004, 110-2811-B-039-507, 110-2320-B-039-048-MY2, 110-2320-B-039-047-MY3, 110-2813-C-039-327-B, 110-2314-B-039-029-MY3, 111-2321-B-006-008, 111-2314-B-039-041-MY3, and 113-2923-B-039-001-MY3/National Science and Technology Council (NSTC)
- ANHRF 110-13, 111-52, 112-24, 112-47, 113-24, 113-38, 113-40/An-Nan Hospital, China Medical University, Tainan, Taiwan
- CMRC-CMA-2/Higher Education Sprout Project by the Ministry of Education (MOE), Taiwan
- ; CMU 110-AWARD-02, 110-N-17, 111-SR-73/China Medical University, Taichung, Taiwan
- DMR-110-124, 111-245, 112-097, 112-086, 112-109, 112-232, DMR-HHC-109-11, HHC-109-12, HHC-110-10, and HHC-111-8/China Medical University Hospital, Taichung, Taiwan.
LinkOut - more resources
Full Text Sources
Medical
Research Materials

