Synthesis and Biological Activity of Chromeno[3,2- c]Pyridines
- PMID: 39519637
- PMCID: PMC11547192
- DOI: 10.3390/molecules29214997
Synthesis and Biological Activity of Chromeno[3,2- c]Pyridines
Abstract
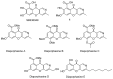
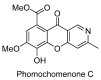
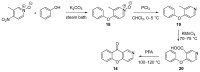
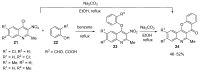
The review summarizes all synthetic methodologies for the preparation of chromeno[3,2-c]pyridines and chromeno[3,2-c]quinolines. The proposed approaches are systemized based on ways for the construction of the heterocyclic system. The presence of these compounds in nature and their bioactivity are also discussed. Natural products with an annelated chromeno[3,2-c]pyridine fragment are well-known and a number of alkaloids derived from this system as a key core have been recently isolated. These compounds demonstrate antimicrobial, antivirus, and cytotoxic activities, making chromeno[3,2-c]pyridine structural motifs promising for medicinal chemistry.
Keywords: biological activities; chromeno[3,2-c]pyridines; chromeno[3,2-c]quinolines; chromenopyridines; synthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





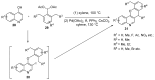







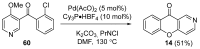
















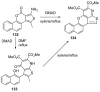




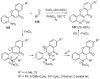


Similar articles
-
Tryptophan derived natural marine alkaloids and synthetic derivatives as promising antimicrobial agents.Eur J Med Chem. 2021 Jan 1;209:112945. doi: 10.1016/j.ejmech.2020.112945. Epub 2020 Oct 21. Eur J Med Chem. 2021. PMID: 33153766 Review.
-
Renieramycin-type alkaloids from marine-derived organisms: Synthetic chemistry, biological activity and structural modification.Eur J Med Chem. 2021 Jan 15;210:113092. doi: 10.1016/j.ejmech.2020.113092. Epub 2020 Dec 9. Eur J Med Chem. 2021. PMID: 33333398 Review.
-
Total Synthesis and Pharmacological Evaluation of Phochrodines A-C.J Nat Prod. 2025 Apr 25;88(4):996-1003. doi: 10.1021/acs.jnatprod.5c00104. Epub 2025 Apr 1. J Nat Prod. 2025. PMID: 40169259 Free PMC article.
-
Sources, Transformations, Syntheses, and Bioactivities of Monoterpene Pyridine Alkaloids and Cyclopenta[c]pyridine Derivatives.Molecules. 2022 Oct 24;27(21):7187. doi: 10.3390/molecules27217187. Molecules. 2022. PMID: 36364013 Free PMC article. Review.
-
Recent advances in isolation, synthesis, and evaluation of bioactivities of bispyrroloquinone alkaloids of marine origin.Chin J Nat Med. 2015 Aug;13(8):561-77. doi: 10.1016/S1875-5364(15)30052-2. Chin J Nat Med. 2015. PMID: 26253489 Free PMC article. Review.
References
-
- Benny A.T., Arikkatt S.D., Vazhappilly C.G., Kannadasan S., Thomas R., Leelabaiamma M.S.N., Radhakrishnan E.K., Shanmugam P. Chromone, A Privileged Scaffold in Drug Discovery: Developments in the Synthesis and Bioactivity. MRMC. 2022;22:1030–1063. doi: 10.2174/1389557521666211124141859. - DOI - PubMed
-
- Verma N., Sood P., Singh J., Jha N.K., Rachamalla M., Dua K. Chromene and its Importance: Chemistry and Biology. In: Kumar Dash A., Kumar D., editors. The Role of Chromenes in Drug Discovery and Development. Bentham Science Publishers; Sharjah, United Arab Emirates: 2023. pp. 1–16.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

