Reactions of an Anionic Gallylene with Azobenzene or Azide Compounds Through C(sp2)-H and C(sp3)-H Activation
- PMID: 39519661
- PMCID: PMC11547653
- DOI: 10.3390/molecules29215021
Reactions of an Anionic Gallylene with Azobenzene or Azide Compounds Through C(sp2)-H and C(sp3)-H Activation
Abstract
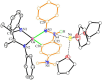
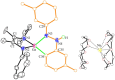
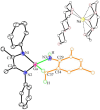
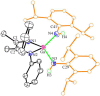
The activation of inert C-H bonds remains a challenge in current chemistry. Here, we report the excellent reactivity of the anionic gallylene species [LGa:][Na(THF)3] (L = [(2,6-iPr2C6H3)NC(CH3)]22-, 1) that allows the selective activation one ortho sp2 C-H bond of several azobenzene and azide derivatives at ambient temperature, with the transfer of the hydrogen atom to one of the nitrogen atoms. The process leads to the formation of the aryl amido products [LGa-κ2N,C-PhNN(H)(p-R-C6H3)][Na(solvent)3] (2, R = H solvent = DME (1,2-Dimethoxyethane); 3, R = -OMe, solvent = DME; 4, R = -NMe2 solvent = THF), [LGa-κ2N,C-(m-CH3-C6H4)NN(H)(m-CH3-C6H3)][Na(15-C-5)2] (5) with new Ga-C and Ga-N bonds. Moreover, 1 is also effective for the C-H activation of two azides RN3 (R = 2,4,6-Me3C6H2 or 2,6-iPr2C6H3), resulting in the formation of gallium amides [LGa(NH-2-(CH2)-4,6-Me2C6H2)][Na(15-C-5)2] (6) and [LGa(NH-2,6-iPr2C6H3)2][Na(THF)5] (7) through intra- or intermolecular sp3 C-H amination. Significantly, these reactions occur for the highly challenging activation of inert C(sp2)-H and C(sp3)-H bonds, thus demonstrating the excellent reactivity of the Ga(I) species 1. The products 2-7 were characterized by X-ray crystallography, 1H and 13C NMR, UV-vis spectroscopy, and density functional theory (DFT) calculations.
Keywords: C–H activation; azobenzene; gallylene; organic azides; α-diimine ligands.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Reactions of Low-Valent Gallium Species with Organic Azides: Formation of Imido-, Azoimido-, and Tetrazene Complexes.Inorg Chem. 2023 Apr 24;62(16):6288-6296. doi: 10.1021/acs.inorgchem.2c04297. Epub 2023 Apr 10. Inorg Chem. 2023. PMID: 37036292
-
Cycloaddition versus Cleavage of the C=S Bond of Isothiocyanates Promoted by Digallane Compounds with Noninnocent α-Diimine Ligands.Chemistry. 2018 Oct 9;24(56):14994-15002. doi: 10.1002/chem.201802469. Epub 2018 Sep 7. Chemistry. 2018. PMID: 30016556
-
Probing the metallating ability of a polybasic sodium alkylmagnesiate supported by a bulky bis(amido) ligand: deprotomagnesiation reactions of nitrogen-based aromatic substrates.Dalton Trans. 2014 Mar 21;43(11):4361-9. doi: 10.1039/c3dt52639a. Dalton Trans. 2014. PMID: 24212265
-
Activation of CO2, CS2, and COS by α-Diimine-Stabilized Gallylenes.Chemistry. 2025 Jan 22;31(5):e202403652. doi: 10.1002/chem.202403652. Epub 2024 Dec 1. Chemistry. 2025. PMID: 39579120
-
Metal-catalyzed silylation of sp3C-H bonds.Chem Soc Rev. 2021 Apr 26;50(8):5062-5085. doi: 10.1039/d0cs01392g. Chem Soc Rev. 2021. PMID: 33629997 Review.
References
Grants and funding
- 22350410394/Instructions for the Research Plan of Research Fund for International Scientists of National Natural Science Foundation of China
- 22171222/The investigations were supported by the National Natural Science Foundation of China
- 2021JM-307, KLSNFM2020004, 19JS065/Natural Science Foundation of Shannxi Province
- 20222159/Ministry of Education basic subject talent training plan 2.0
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

