Synthesis of Perfluoroalkylated Pyrazoles from α-Perfluoroalkenylated Aldehydes
- PMID: 39519675
- PMCID: PMC11547949
- DOI: 10.3390/molecules29215034
Synthesis of Perfluoroalkylated Pyrazoles from α-Perfluoroalkenylated Aldehydes
Abstract
Within this study, we report a simple two-step process for the synthesis of perfluoroalkylated pyrazoles from aliphatic aldehydes. In the photocatalytic first step, the aldehydes are transformed into the corresponding perfluoroalkylated enals, which then undergo nucleophilic attack by hydrazine and subsequent ring closure, providing the fluorinated 3,4-substituted pyrazole products in a 64-84% yield. Using triphenylphosphine and imidazolidinone as organocatalysts, the method is operationally simple and omits heavy metal-containing waste.
Keywords: aldehydes; heterocycles; perfluoroalkylations; photocatalysis; pyrazoles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



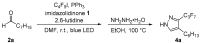

Similar articles
-
Straightforward Synthesis of Fluorinated Enals via Photocatalytic α-Perfluoroalkenylation of Aldehydes.J Org Chem. 2021 Jun 4;86(11):7425-7438. doi: 10.1021/acs.joc.1c00383. Epub 2021 May 19. J Org Chem. 2021. PMID: 34008975
-
Synthesis and Antibacterial Screening of Some Pyrazole Derivatives Catalyzed by Cetyltrimethylammoniumbromide (CTAB).Curr Org Synth. 2021;18(2):225-231. doi: 10.2174/1570179417666200620220232. Curr Org Synth. 2021. PMID: 32562527
-
Highly regioselective organocatalyzed synthesis of pyrazoles from diazoacetates and carbonyl compounds.Chemistry. 2013 Jun 3;19(23):7555-60. doi: 10.1002/chem.201300047. Epub 2013 Apr 10. Chemistry. 2013. PMID: 23576283
-
Organocatalysis Combined with Photocatalysis.Top Curr Chem (Cham). 2019 Nov 15;377(6):37. doi: 10.1007/s41061-019-0265-0. Top Curr Chem (Cham). 2019. PMID: 31728771 Review.
-
Synthesis of Chromone-Related Pyrazole Compounds.Molecules. 2017 Oct 5;22(10):1665. doi: 10.3390/molecules22101665. Molecules. 2017. PMID: 28981465 Free PMC article. Review.
References
-
- Ansari A., Ali A., Asif M., Shamsuzzaman S. Review: Biologically active pyrazole derivatives. New J. Chem. 2017;41:16–41. doi: 10.1039/C6NJ03181A. - DOI
-
- Steinbach G., Lynch P.M., Phillips R.K., Wallace M.H., Hawk E., Gordon G.B., Wakabayashi N., Saunders B., Shen Y., Fujimura T., et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 2000;342:26. doi: 10.1056/NEJM200006293422603. - DOI - PubMed
-
- Uslaner J.M., Parmentier-Batteur S., Flick R.B., Surles N.O., Lam J.S.H., McNaughton C.H., Jacobson M.A., Hutson P.H. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

