Synthesis of 6- or 8-Carboxamido Derivatives of Imidazo[1,2- a]pyridines via a Heterogeneous Catalytic Aminocarbonylation Reaction
- PMID: 39519688
- PMCID: PMC11547779
- DOI: 10.3390/molecules29215048
Synthesis of 6- or 8-Carboxamido Derivatives of Imidazo[1,2- a]pyridines via a Heterogeneous Catalytic Aminocarbonylation Reaction
Abstract
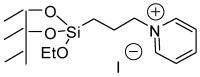
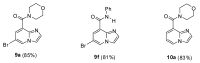
Imidazo[1,2-a]pyridines and especially their amide derivatives exhibit a wide range of favourable pharmacological properties. In this work, Pd-catalysed carbonylation was used for the first time for the introduction of the carboxamide moiety into positions 6 or 8. A recyclable Pd catalyst, with palladium immobilised on a supported ionic liquid phase decorated with pyridinium ions, was used efficiently for the conversion of 6- or 8-iodo derivatives to the products. In the case of 6-iodo derivatives, a competing mono- and double carbonylation could be observed in the reactions of aliphatic amines as nucleophiles, but under the proper choice of reaction conditions, good-to-excellent selectivities could be achieved towards either the corresponding amides or α-ketomides. The heterogeneous catalyst showed excellent recyclability and low Pd-leaching.
Keywords: amide; palladium catalyst; supported ionic liquid; α-ketoamide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Mishra N.P., Mohapatra S., Das T., Nayak S. Imidazo[1,2-a]pyridine as a promising scaffold for the development of antibacterial agents. J. Heterocycl. Chem. 2022;59:2051–2075. doi: 10.1002/jhet.4534. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

