Identification of a New Promising BAG3 Modulator Featuring the Imidazopyridine Scaffold
- PMID: 39519692
- PMCID: PMC11547576
- DOI: 10.3390/molecules29215051
Identification of a New Promising BAG3 Modulator Featuring the Imidazopyridine Scaffold
Abstract
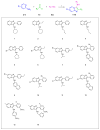
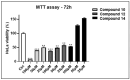
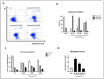
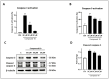
The antiapoptotic BAG3 protein plays a crucial role in cellular proteostasis and it is involved in several signalling pathways governing cell proliferation and survival. Owing to its multimodular structure, it possesses an extensive interactome including the molecular chaperone HSP70 and other specific cellular partners, which make it an eminent factor in several pathologies, particularly in cancer. Despite its potential as a therapeutic target, very few BAG3 modulators have been disclosed so far. Here we describe the identification of a promising BAG3 modulator able to bind the BAG domain of the protein featuring an imidazopyridine scaffold and obtained through the application of the Groebke-Blackburn-Bienaymé chemical synthesis procedure. The disclosed compound 10 showed a relevant cytotoxic activity, and in line with the biological profile of BAG3 disruption, it induced the activation of caspase 3 and 9.
Keywords: BAG domain modulator; BAG3 protein; Groebke–Blackburn–Bienaymé reaction; Surface Plasmon Resonance assay; imidazopyridine scaffold.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

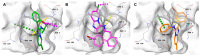
Similar articles
-
Identification of the first-in-class dual inhibitor targeting BAG3 and HSP70 proteins to disrupt multiple chaperone pathways.Eur J Med Chem. 2025 Apr 5;287:117358. doi: 10.1016/j.ejmech.2025.117358. Epub 2025 Feb 6. Eur J Med Chem. 2025. PMID: 39947053
-
Anti-tumor compounds identification from gossypol Groebke imidazopyridine product.Bioorg Chem. 2021 Sep;114:105146. doi: 10.1016/j.bioorg.2021.105146. Epub 2021 Jul 5. Bioorg Chem. 2021. PMID: 34328859
-
Structural Refinement of 2,4-Thiazolidinedione Derivatives as New Anticancer Agents Able to Modulate the BAG3 Protein.Molecules. 2022 Jan 20;27(3):665. doi: 10.3390/molecules27030665. Molecules. 2022. PMID: 35163936 Free PMC article.
-
Breaking BAG: The Co-Chaperone BAG3 in Health and Disease.Trends Pharmacol Sci. 2016 Aug;37(8):672-688. doi: 10.1016/j.tips.2016.04.007. Epub 2016 May 6. Trends Pharmacol Sci. 2016. PMID: 27162137 Review.
-
Corelating the molecular structure of BAG3 to its oncogenic role.Cell Biol Int. 2024 Aug;48(8):1080-1096. doi: 10.1002/cbin.12199. Epub 2024 Jun 23. Cell Biol Int. 2024. PMID: 38924608 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

