Beware of N-Benzoyloxybenzamides
- PMID: 39519784
- PMCID: PMC11548001
- DOI: 10.3390/molecules29215143
Beware of N-Benzoyloxybenzamides
Abstract
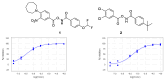
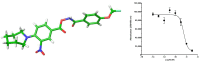
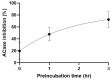
Following a High-Throughput Screening campaign to discover inhibitors of acid ceramidase, we report the novel and extremely potent covalent inhibitor, 1. Following resynthesis and stability monitoring, we discovered that 1 is chemically unstable and reacts with DMSO at room temperature. This mode of decomposition is likely general for this class of compound, and we urge caution for their use in drug discovery research.
Keywords: N-benzoyloxybenzamides; PAINS; acid ceramidase.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


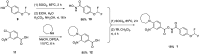


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

