Design, Synthesis and Biological Activity Evaluation of β-Carboline Derivatives Containing Nitrogen Heterocycles
- PMID: 39519796
- PMCID: PMC11547513
- DOI: 10.3390/molecules29215155
Design, Synthesis and Biological Activity Evaluation of β-Carboline Derivatives Containing Nitrogen Heterocycles
Abstract
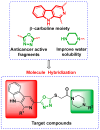
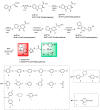
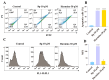
A series of β-carboline derivatives containing nitrogen heterocycles were designed and synthesized. All compounds were screened for their antitumor activity against four human tumor cell lines (A549, K562, PC-3, T47D). Notably, compound N-(4-(morpholinomethyl)phenyl)-2-((5-(1-(3,4,5-trimethoxyphenyl)-9H-pyrido[3,4-b]indol-3-yl)-1,3,4-oxadiazol-2-yl)thio)acetamide (8q) exhibited significant inhibitory activity against PC-3 cells with an IC50 value of 9.86 µM. Importantly, compound 8q effectively suppressed both the proliferation and migration of PC-3 cells. Mechanistic studies revealed that compound 8q induced cell apoptosis and caused the accumulation of reactive oxygen species (ROS), leading to cell cycle arrest in the G0/G1 phase in PC-3 cells.
Keywords: ROS; antitumor; apoptosis; cell cycle; β-carboline derivatives.
Conflict of interest statement
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Design and synthesis of β-carboline derivatives with nitrogen mustard moieties against breast cancer.Bioorg Med Chem. 2021 Sep 1;45:116341. doi: 10.1016/j.bmc.2021.116341. Epub 2021 Aug 2. Bioorg Med Chem. 2021. PMID: 34365102
-
Design and Synthesis of DNA-Interactive β-Carboline-Oxindole Hybrids as Cytotoxic and Apoptosis-Inducing Agents.ChemMedChem. 2018 Sep 19;13(18):1909-1922. doi: 10.1002/cmdc.201800402. Epub 2018 Aug 22. ChemMedChem. 2018. PMID: 30010248
-
Synthesis, characterization, cellular uptake and apoptosis-inducing properties of two highly cytotoxic cyclometalated ruthenium(II) β-carboline complexes.Eur J Med Chem. 2017 Nov 10;140:104-117. doi: 10.1016/j.ejmech.2017.09.007. Epub 2017 Sep 6. Eur J Med Chem. 2017. PMID: 28923379
-
Synthesis and biological evaluation of novel 3,9-substituted β-carboline derivatives as anticancer agents.Bioorg Med Chem Lett. 2015 Sep 15;25(18):3873-7. doi: 10.1016/j.bmcl.2015.07.058. Epub 2015 Jul 26. Bioorg Med Chem Lett. 2015. PMID: 26235951
-
Exploring β-carboline hybrids and their derivatives: A review on synthesis and anticancer efficiency.Eur J Med Chem. 2025 Apr 15;288:117412. doi: 10.1016/j.ejmech.2025.117412. Epub 2025 Feb 17. Eur J Med Chem. 2025. PMID: 39987835 Review.
Cited by
-
Photo-induced decarboxylative radical cascade cyclization of unactivated alkenes: access to CF- and CF2-substituted ring-fused imidazoles.RSC Adv. 2025 Apr 22;15(16):12739-12745. doi: 10.1039/d5ra02023a. eCollection 2025 Apr 16. RSC Adv. 2025. PMID: 40264862 Free PMC article.
References
-
- Ling Y., Xu C., Luo L., Cao J., Feng J., Xue Y., Zhu Q., Ju C., Li F., Zhang Y., et al. Novel β-Carboline/Hydroxamic Acid Hybrids Targeting Both Histone Deacetylase and DNA Display High Anticancer Activity via Regulation of the p53 Signaling Pathway. J. Med. Chem. 2015;58:9214–9227. doi: 10.1021/acs.jmedchem.5b01052. - DOI - PubMed
-
- Sathish M., Chetan Dushantrao S., Nekkanti S., Tokala R., Thatikonda S., Tangella Y., Srinivas G., Cherukommu S., Hari Krishna N., Shankaraiah N., et al. Synthesis of DNA interactive C3-trans-cinnamide linked β-carboline conjugates as potential cytotoxic and DNA topoisomerase I inhibitors. Bioorgan. Med. Chem. 2018;26:4916–4929. doi: 10.1016/j.bmc.2018.08.031. - DOI - PubMed
-
- Sathish M., Kavitha B., Nayak V.L., Tangella Y., Ajitha A., Nekkanti S., Alarifi A., Shankaraiah N., Nagesh N., Kamal A. Synthesis of podophyllotoxin linked β-carboline congeners as potential anticancer agents and DNA topoisomerase II inhibitors. Eur. J. Med. Chem. 2018;144:557–571. doi: 10.1016/j.ejmech.2017.12.055. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

