Improved Protocol for Efficient Agrobacterium-Mediated Transient Gene Expression in Medicago sativa L
- PMID: 39519910
- PMCID: PMC11547841
- DOI: 10.3390/plants13212992
Improved Protocol for Efficient Agrobacterium-Mediated Transient Gene Expression in Medicago sativa L
Abstract
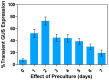
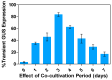
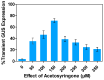
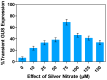
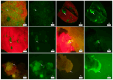
Medicago sativa L. (Alfalfa) is a globally recognized forage legume that has recently gained attention for its high protein content, making it suitable for both human and animal consumption. However, due to its perennial nature and autotetraploid genetics, conventional plant breeding requires a longer timeframe compared to other crops. Therefore, genetic engineering offers a faster route for trait modification and improvement. Here, we describe a protocol for achieving efficient transient gene expression in alfalfa through genetic transformation with the Agrobacterium tumefaciens pCAMBIA1304 vector. This vector contains the reporter genes β-glucuronidase (GUS) and green fluorescent protein (GFP), along with a selectable hygromycin B phosphotransferase gene, all driven by the CaMV 35s promoter. Various transformation parameters-such as different explant types, leaf ages, leaf sizes, wounding types, bacterial concentrations (OD600nm), tissue preculture periods, infection periods, co-cultivation periods, and different concentrations of acetosyringone, silver nitrate, and calcium chloride-were optimized using 3-week-old in vitro-grown plantlets. Results were attained from data based on the semi-quantitative observation of the percentage and number of GUS spots on different days of agro-infection in alfalfa explants. The highest percentage of GUS positivity (76.2%) was observed in 3-week-old, scalpel-wounded, segmented alfalfa leaf explants after 3 days of agro-infection at a bacterial concentration of 0.6, with 2 days of preculture, 30 min of co-cultivation, and the addition of 150 µM acetosyringone, 4 mM calcium chloride, and 75 µM silver nitrate. The transient expression of genes of interest was confirmed via histochemical GUS and GFP assays. The results based on transient reporter gene expression suggest that various factors influence T-DNA delivery in the Agrobacterium-mediated transformation of alfalfa. The improved protocol can be used in stable transformation techniques for alfalfa.
Keywords: Agrobacterium tumefaciens; Medicago sativa L. (alfalfa); green fluorescent protein (GFP); transient gene; β-glucuronidase (GUS).
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures










Similar articles
-
Evaluation of Parameters Affecting Agrobacterium-Mediated Transient Gene Expression in Industrial Hemp (Cannabis sativa L.).Plants (Basel). 2024 Feb 28;13(5):664. doi: 10.3390/plants13050664. Plants (Basel). 2024. PMID: 38475511 Free PMC article.
-
Establishment of Agrobacterium tumefaciens - mediated genetic transformation of apple pathogen Marssonina coronaria using marker genes under the control of CaMV 35S promoter.Microbiol Res. 2021 Dec;253:126878. doi: 10.1016/j.micres.2021.126878. Epub 2021 Sep 24. Microbiol Res. 2021. PMID: 34607236
-
Assessment of factors affecting Agrobacterium-mediated genetic transformation of the unicellular green alga, Chlorella vulgaris.World J Microbiol Biotechnol. 2012 Apr;28(4):1771-9. doi: 10.1007/s11274-011-0991-0. Epub 2011 Dec 29. World J Microbiol Biotechnol. 2012. PMID: 22805959
-
Agrobacterium-mediated genetic transformation of Pogostemon cablin (Blanco) Benth. Using leaf explants: bactericidal effect of leaf extracts and counteracting strategies.Appl Biochem Biotechnol. 2012 Apr;166(8):1871-95. doi: 10.1007/s12010-012-9612-0. Epub 2012 Mar 21. Appl Biochem Biotechnol. 2012. PMID: 22434351
-
Alfalfa (Medicago sativa L.): literature quantitative research analysis.Nat Prod Res. 2025 May 9:1-10. doi: 10.1080/14786419.2025.2486335. Online ahead of print. Nat Prod Res. 2025. PMID: 40340622 Review.
Cited by
-
Reprogramming Hairy Root Cultures: A Synthetic Biology Framework for Precision Metabolite Biosynthesis.Plants (Basel). 2025 Jun 23;14(13):1928. doi: 10.3390/plants14131928. Plants (Basel). 2025. PMID: 40647937 Free PMC article. Review.
References
-
- Yazicilar B., Chang Y.L. Embryogenic callus differentiation in short-term callus derived from leaf explants of alfalfa cultivars. Nat. Prod. Biotechnol. 2022;2:99–104. doi: 10.58465/natprobiotech.2022.10. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

