Induction and Characteristics of Callus Cultures of the Medicinal Plant Tussilago farfara L
- PMID: 39519998
- PMCID: PMC11548631
- DOI: 10.3390/plants13213080
Induction and Characteristics of Callus Cultures of the Medicinal Plant Tussilago farfara L
Abstract
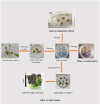
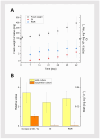
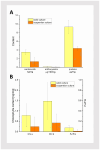
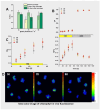
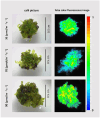
Tussilago farfara L. is a traditional medicinal plant valued for its potentially health-promoting metabolites. Its herbal raw material has been recognized and used since ancient times and continues to be widely used in traditional medicine. Introducing this plant species to in vitro cultivation is a challenging task, but once the protocol is developed, such cultures can provide an abundant and inexhaustible source of plant material. In this study, we report the successful induction and growth of vigorous T. farfara callus in vitro. Callus induction was achieved on MS solid media with the combination of indole-3-acetic acid (3 mg/L) and benzyl aminopurine (2 mg/L) in darkness, whereas it appeared inefficient under light conditions and in suspension culture. We present a detailed description of callus growth kinetics, morphological analysis, photosynthetic activity, and biochemical parameters (including protein content and photosynthetic pigments) supported by histological studies. Furthermore, we observed the potential for organogenesis and somatic embryogenesis. This method for the in vitro propagation of T. farfara, along with callus culture maintenance, offers a wide range of applications in pharmacy for the production of valuable metabolites. Moreover, it could benefit the environment by reducing the depletion of natural populations of this species and may serve as an alternative strategy for species conservation in light of global warming.
Keywords: carotenoids; chlorophyll; coltsfoot; fluorescence; histology; organogenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
[Tissue culture of callus and establishment of regeneration system of Tussilago farfara petiole].Zhongguo Zhong Yao Za Zhi. 2017 Oct;42(20):3895-3900. doi: 10.19540/j.cnki.cjcmm.2017.0156. Zhongguo Zhong Yao Za Zhi. 2017. PMID: 29243424 Chinese.
-
A review of the ethnobotanical value, phytochemistry, pharmacology, toxicity and quality control of Tussilago farfara L. (coltsfoot).J Ethnopharmacol. 2021 Mar 1;267:113478. doi: 10.1016/j.jep.2020.113478. Epub 2020 Oct 16. J Ethnopharmacol. 2021. PMID: 33069788 Free PMC article. Review.
-
A Comparative Analysis of the Anatomy, Phenolic Profile, and Antioxidant Capacity of Tussilago farfara L. Vegetative Organs.Plants (Basel). 2022 Jun 23;11(13):1663. doi: 10.3390/plants11131663. Plants (Basel). 2022. PMID: 35807614 Free PMC article.
-
Mass propagation through direct and indirect organogenesis in three species of genus Zephyranthes and ploidy assessment of regenerants through flow cytometry.Mol Biol Rep. 2021 Jan;48(1):513-526. doi: 10.1007/s11033-020-06083-1. Epub 2021 Jan 13. Mol Biol Rep. 2021. PMID: 33442831
-
Biotechnology for propagation and secondary metabolite production in Bacopa monnieri.Appl Microbiol Biotechnol. 2022 Mar;106(5-6):1837-1854. doi: 10.1007/s00253-022-11820-6. Epub 2022 Feb 26. Appl Microbiol Biotechnol. 2022. PMID: 35218388 Review.
Cited by
-
Adsorption of Methylene Blue onto Environmentally Friendly Lignocellulosic Material Obtained from Mature Coltsfoot (Tussilago farfara) Leaves.Polymers (Basel). 2025 Jun 2;17(11):1549. doi: 10.3390/polym17111549. Polymers (Basel). 2025. PMID: 40508793 Free PMC article.
References
-
- [(accessed on 19 September 2024)]. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:256904-1.
-
- Tobyn G., Denham A., Whitelegg M., editors. Medical Herbs. Churchill Livingstone; Edinburgh, UK: 2011. CHAPTER 30—Tussilago farfara, coltsfoot; pp. 317–326. - DOI
-
- Matuszkiewicz W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski. Wydawnictwo Naukowe PWN; Warszawie, Poland: 2006.
-
- Adamczak A., Opala B., Gryszczyńska A., Buchwald W. Content of pyrrolizidine alkaloids in the leaves of coltsfoot (Tussilago farfara L.) in Poland. Acta Soc. Bot. Pol. 2013;82:289–293. doi: 10.5586/asbp.2013.028. - DOI
LinkOut - more resources
Full Text Sources

