Overexpression of Cassava MeSTP7 Promotes Arabidopsis Seedling Development
- PMID: 39520020
- PMCID: PMC11548149
- DOI: 10.3390/plants13213102
Overexpression of Cassava MeSTP7 Promotes Arabidopsis Seedling Development
Abstract
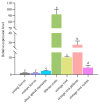
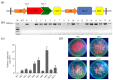
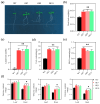
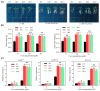
The sugar transporter (STP) gene family is a key regulator of plant development, which is crucial for the efficient transport and utilization of sugars during plant growth and development. In this study, we identified the MeSTP7 gene, which is highly expressed in cassava fibrous roots, early storage roots, and under hormonal treatment, including IAA, MeJA, ABA, and GA3, and abiotic stressors, such as mannitol and NaCl. A strong response was observed with exoqenous IAA. Transfecting MeSTP7 into Arabidopsis promoted early seedling growth, particularly in lateral root development. The content of endogenous hormones (IAA and MeJA) as well as soluble sugars (sucrose, fructose, and glucose) was elevated in transgenic Arabidopsis. Hormone treatments with IAA, MeJA, GA3, and ABA on transgenic Arabidopsis revealed that transgenic Arabidopsis responded positively to added 20 μM IAA. They also exhibited co-induced regulation of lateral root formation by GA3, MeJA, and ABA. qRT-PCR analysis showed that overexpression of MeSTP7 upregulated the expression of IAA14, ARF7, and ARF19 in Arabidopsis. Under IAA treatment, the expression of these genes was similarly upregulated but downregulated under MeJA treatment. These results suggest that MeSTP7 may promote Arabidopsis seedling development by increasing the content of sucrose, glucose, and fructose in roots, which in turn influences IAA-based hormonal signaling.
Keywords: MeSTP7; cassava; hormone treatment; sugar transporter protein; transgenic Arabidopsis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis.Plant J. 2005 Nov;44(3):382-95. doi: 10.1111/j.1365-313X.2005.02537.x. Plant J. 2005. PMID: 16236149
-
Promoter of Cassava MeAHL31 Responds to Diverse Abiotic Stresses and Hormone Signals in Transgenic Arabidopsis.Int J Mol Sci. 2024 Jul 14;25(14):7714. doi: 10.3390/ijms25147714. Int J Mol Sci. 2024. PMID: 39062957 Free PMC article.
-
Multiple AUX/IAA-ARF modules regulate lateral root formation: the role of Arabidopsis SHY2/IAA3-mediated auxin signalling.Philos Trans R Soc Lond B Biol Sci. 2012 Jun 5;367(1595):1461-8. doi: 10.1098/rstb.2011.0232. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22527388 Free PMC article.
-
Comparison of endogenous hormone content and balance in Pinus yunnanensis Franch. seedlings after decapitation.Front Plant Sci. 2025 Apr 16;16:1531575. doi: 10.3389/fpls.2025.1531575. eCollection 2025. Front Plant Sci. 2025. PMID: 40308297 Free PMC article.
-
LBD18 and IAA14 antagonistically interact with ARF7 via the invariant Lys and acidic residues of the OPCA motif in the PB1 domain.Planta. 2023 Jun 24;258(2):26. doi: 10.1007/s00425-023-04183-3. Planta. 2023. PMID: 37354348
Cited by
-
Xuesanqi ameliorates DSS-induced colitis in mice by mediating gut microbiota dysbiosis and modulating MAPK/ERK/JNK pathway.Nat Prod Bioprospect. 2024 Dec 1;14(1):60. doi: 10.1007/s13659-024-00482-8. Nat Prod Bioprospect. 2024. PMID: 39616288 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

