Population pharmacokinetics and exposure-response relationships of dostarlimab in primary advanced or recurrent endometrial cancer in part 1 of RUBY
- PMID: 39520048
- PMCID: PMC11862793
- DOI: 10.1111/bcp.16325
Population pharmacokinetics and exposure-response relationships of dostarlimab in primary advanced or recurrent endometrial cancer in part 1 of RUBY
Abstract
Aims: Dostarlimab-gxly is a humanized monoclonal antibody of the IgG4 isotype that binds to the programmed cell death protein-1 (PD-1) receptor and blocks its ligands. RUBY (NCT03981796) is a two-part multicentre study in patients with recurrent or primary advanced endometrial cancer. The overall aims were to characterise the population pharmacokinetics (PopPK) from Part 1 of this study, identify relevant covariates of interest, and assess exposure-efficacy/safety (ER) relationships.
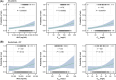
Methods: A PopPK model developed using GARNET (NCT02715284) study data for dostarlimab monotherapy was externally validated with RUBY Part 1 study data. Subsequently, the model was updated with data across the two studies. Exposure-safety analyses for adverse events related to dostarlimab alone or in combination with standard of care (SOC) were modelled using logistic regression. Exposure-efficacy analysis included Cox proportional hazards analysis of the primary efficacy endpoint of progression-free survival (PFS).
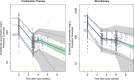
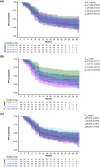
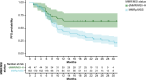
Results: For the model update, 7957 pharmacokinetics observations from 868 patients pooled from both RUBY and GARNET studies were available. The model was consistent with the previous model. Dostarlimab clearance was estimated to be 7.79% lower when dostarlimab was given as SOC combination therapy. However, no significant covariates were clinically relevant. Hepatic or renal impairment did not affect pharmacokinetics. Among the safety endpoints, only rash showed a small yet statistically significant effect (P < .05) in all subjects; however, this was not not deemed clinically relevant. There were no other clinically significant exposure-safety or exposure-PFS relationships.
Conclusions: The addition of chemotherapy to dostarlimab had limited effect on dostarlimab PopPK, with no clinically significant covariates or clinically relevant exposure-safety or exposure-PFS relationships.
Keywords: clinical pharmacology; oncology; pharmacokinetics; therapeutics.
© 2024 The Author(s). British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
M. Kuchimanchi is an employee of GSK and may have stock or stock options in GSK.
T.L. Jørgensen has nothing to disclose.
E. Hanze reports institutional consulting fees from GSK.
T. André reports attending advisory board meetings and receiving consulting fees from AbbVie, Aptitude Health, Bristol Myers Squibb, Gritstone Bio, GamaMabs Pharma, Gilead, Nordic, GSK, Merck & Co., Seagen, Servier and Takeda; honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from Bristol Myers Squibb, Gritstone Bio, GSK, Merck & Co., Merck Serono, Roche/Ventana, Seagen and Servier; support for attending meetings from Bristol Myers Squibb and Merck & Co.; data safety monitoring board for NSPIRNA: Phase 2 randomized controlled trial of ompenaclid in 2nd line RAS mutant metastatic colorectal cancer; and leadership or fiduciary role in ARCAD foundation (Aide à la recherché en cancérologie digestive).
A. Jain has nothing to disclose.
D. Berton has nothing to disclose.
O. Alskär reports institutional consulting fees from GSK.
O. Zub has nothing to disclose.
A. Oaknin reports institutional grants from AbbVie Deutschland, Advaxis, Aeterna Zentaris, Amgen, Aprea Therapeutics, Bristol Myers Squibb, Clovis Oncology, Eisai, Roche, ImmunoGen, MSD de España, Millennium Pharmaceuticals, PharmaMar, Regeneron and Tesaro; consulting fees from Agenus, AstraZeneca, Clovis Oncology, Corcept Therapeutics, Deciphera Pharmaceuticals, Eisai, EMD Serono, Exelixis, Roche, Genmab, GSK, ImmunoGen, iTeos, MSD de España, Mersana Therapeutics, Novocure, OncXerna Therapeutics, PharmaMar, Regeneron, Seagen, Shattuck Labs and Sutro Biopharma; honoraria from Asociación Colombiada de Ginecológos Oncólogos, AstraZeneca, ESO, GSK, Medscape, NSGO, PeerView and PeerVoice; individual travel support from AstraZeneca, PharmaMar and Roche; and advisory board participation for Agenus, AstraZeneca, Clovis Oncology, Corcept Therapeutics, Deciphera Pharmaceuticals, Eisai, EMD Serono, Exelixis, Roche, Genmab, GSK, ImmunoGen, iTeos, Mersana Therapeutics, MSD de España, Novocure, OncXerna Therapeutics, PharmaMar, Regeneron, Seagen, Shattuck Labs and Sutro Biopharmav.
M.A. Shahin reports institutional grants from AstraZeneca, GSK and Merck; honoraria from AstraZeneca, GSK, Merck and Seagen; expert testimony fees from Robinson & Havens PSC, Lexington KY; advisory board fees from Seagen; and board member for Unite for Her.
A. Koliadi reports consulting fees from Eisai and GSK.
B. Pothuri reports institutional grant support from AstraZeneca, Celsion, Clovis Oncology, Duality Bio, Eisai, Genentech/Roche, Karyopharm Therapeutics, Merck, Mersana Therapeutics, Onconova Therapeutics, Seagen, Sutro Biopharma, Takeda, Tesaro/GSK, Toray and VBL Therapeutics and consulting fees from AstraZeneca, BioNTech, Clovis Oncology, Eisai, GOG Foundation, Lily, Merck, Mersana Therapeutics, Onconova Therapeutics, Sutro Biopharma, Tesaro/GSK and Toray.
T. Krivak reports consulting and speakers' bureau fees from AstraZeneca, GSK, ImmunoGen, Merck, Myriad Genetics and Seagen/Genmab and research support from AstraZeneca, GSK and ImmunoGen.
M. Pishchyk has nothing to disclose.
Y. Segev has nothing to disclose.
F.J. Backes reports advisory board consulting fees for Merck, GSK, Eisai, Clovis Oncology, ImmunoGen, Myriad Genetics, AstraZeneca, EMD Serono, Daiichi Sankyo and BioNTech.
C. Gennigens reports grants/contracts from AstraZeneca and GSK; consulting fees from GSK, Ipsen and MSD; honoraria from AstraZeneca, Bristol Myers Squibb, Ipsen, MSD, Pfizer and PharmaMar; support for attending meetings from GSK, Ipsen, MSD, Pfizer and PharmaMar; and participation on a data safety monitoring or advisory board for AstraZeneca, Bristol Myers Squibb, Eisai, GSK, Ipsen and MSD.
S. Bouberhan reports consulting fees from ImmunoGen.
S. Zajic is an employee of GSK and reports stock at GSK.
M. Melham is a full‐time employee of GSK and owns stocks/shares.
J. Buscema has nothing to disclose.
Figures

References
-
- Oaknin A, Tinker AV, Gilbert L, et al. Clinical activity and safety of the anti‐programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair‐deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol. 2020;6(11):1766‐1772. doi:10.1001/jamaoncol.2020.4515 - DOI - PMC - PubMed
-
- GSK . Jemperli package insert. 2024. Accessed August 2, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761174s009lbl.pdf
-
- GSK . Jemperli Summary of Product Characteristics. GSK; 2024. Accessed September 5, 2024. https://www.ema.europa.eu/en/documents/product-information/jemperli-epar...
-
- National Institute for Health and Care Excellence . Dostarlimab for previously treated advanced or recurrent endometrial cancer with high microsatellite instability or mismatch repair deficiency. 2024. Accessed September 5, 2024. Accessed September 5, 2024. https://www.nice.org.uk/guidance/ta963/resources/dostarlimab‐with‐platin...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

