Biologics and airway remodeling in asthma: early, late, and potential preventive effects
- PMID: 39520155
- PMCID: PMC11804314
- DOI: 10.1111/all.16382
Biologics and airway remodeling in asthma: early, late, and potential preventive effects
Abstract
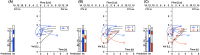
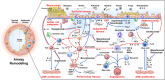
Although airway remodeling in severe and/or fatal asthma is still considered irreversible, its individual components as a cause of clinical symptoms and/or lung function changes remain largely unknown. While inhaled glucocorticoids have not consistently been shown to affect airway remodeling, biologics targeting specific pathways of airway inflammation have been shown to improve lung function, mucus plugging, and airway structural changes that can exceed those seen with glucocorticoids. This superiority of biologic treatment, which cannot be solely explained by insufficient doses or limited durations of glucocorticoid therapies, needs to be further explored. For this field of research, we propose a novel classification of the potential effects of biologics on airway remodeling into three temporal effects: early effects (days to weeks, primarily modulating inflammatory processes), late effects (months to years, predominantly affecting structural changes), and potential preventive effects (outcomes of early treatment with biologics). For the identification of potential preventive effects of biologics, we call for studies exploring the impact of early biological treatment on airway remodeling in patients with moderate-to-severe asthma, which should be accompanied by a long-term evaluation of clinical parameters, biomarkers, treatment burden, and socioeconomic implications.
Keywords: asthma treatment; biologics; remodeling.
© 2024 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
GV reports research support from AstraZeneca. RP has no potential conflicts of interest to declare. ML has received consulting fees or honoraria, or both for lectures from ALK, Allergopharma, AstraZeneca, Berlin‐Chemie, Boehringer Ingelheim, Chiesi, GSK, HAL Allergy, Leti, Novartis, MSD, Sanofi, and TEVA; and grants for research or clinical trials, or both from Deutsche Forschungsgemeinschaft, AstraZeneca, and GSK. GB reports personal fees from Astra Zeneca, personal fees from Boehringer‐Ingelheim, personal fees from Chiesi, personal fees from GSK, personal fees from Novartis, personal fees from Sanofi, grants from MSD, outside the submitted work. G.W.C. has received consulting fees or honoraria, or both for lectures from AstraZeneca, Chiesi, Novartis, Sanofi, Menarini, Stallergenes Greer, GSK, and HAL Allergy. FB has been on the scientific Board for AZ, BI, Chiesi, GSK, Menarini group, Sanofi and P&G; received honoraria from AZ, BI, Chiesi, GSK, Menarini group and Sanofi. JCV is a full time employee of the University of Rostock as a full time professor and chair of the Departments of Pneumology and Intensive Care Medicine has given independent advice, lectured for and received honoraria from AstraZeneca, Avontec, Bayer, Bencard, Bionorica, Boehringer‐Ingelheim, Chiesi, Essex/Schering‐Plough, GSK, Janssen‐Cilag, Leti, MEDA, Merck, MSD, Mundipharma, Novartis, Nycomed/Altana, Pfizer, Revotar, Sandoz‐Hexal, Stallergens, TEVA, UCB/Schwarz‐Pharma, Zydus/Cadila, has participated in advisory boards for Avontec, Boehringer‐Ingelheim, Chiesi, Essex/Schering‐Plough, GSK, Janssen‐Cilag, MEDA, MSD, Mundipharma, Novartis, Regeneron, Revotar, Roche, Sanofi‐Aventis, Sandoz‐Hexal, TEVA, UCB/Schwarz‐Pharma and has received research grants from the Deutsche Forschungsgesellschaft, Land Mecklenburg‐Vorpommern, GSK, MSD. G.W.C. received honoraria for lectures, presentations, speakers from AstraZeneca, GSK, Novartis, Sanofi, Stallergenes, Greer, Hal Allergy, Menarini, Chiesi, Mylan, Valeas, Faes.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

