TICA-CLOP STUDY: Ticagrelor Versus Clopidogrel in Acute Moderate and Moderate-to-Severe Ischemic Stroke, a Randomized Controlled Multi-Center Trial
- PMID: 39520630
- PMCID: PMC11695443
- DOI: 10.1007/s40263-024-01127-7
TICA-CLOP STUDY: Ticagrelor Versus Clopidogrel in Acute Moderate and Moderate-to-Severe Ischemic Stroke, a Randomized Controlled Multi-Center Trial
Abstract
Background: Many studies evaluated the efficacy and safety of ticagrelor versus clopidogrel in patients with ischemic stroke; none of these trials included North African participants, and all of these trials comprised only participants who experienced transient ischemic attack (TIA) or minor stroke.
Objectives: We compared the efficacy and safety of ticagrelor versus clopidogrel in patients with first-ever noncardioembolic moderate or moderate-to-severe ischemic stroke.
Methods: Our trial involved 900 first-ever noncardioembolic patients with acute ischemic stroke (AIS) who randomly received either loading and maintenance doses of ticagrelor or clopidogrel within the first 24 h of stroke onset.
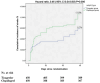
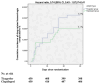
Results: We involved 900 patients in the intention-to-treat analysis. A total of 39 (8.7%) patients in ticagrelor arm and 62 (13.8%) in clopidogrel arm experienced a new stroke [hazard ratio (HR) 0.46; 95% confidence interval (CI) 0.34-0.83; P value = 0.006]. A total of 57 (12.7%) patients in ticagrelor group and 80 (17.8%) patients in clopidogrel group experienced composite of new stroke, myocardial infarction (MI), or death due to vascular insults (HR 0.51; 95% CI 0.43-0.82; P value = 0.004). Participants who received ticagrelor experienced less frequent unfavorable outcomes. We found no significant variation between our study's two arms concerning the hemorrhagic and non-hemorrhagic complications.
Conclusion: Patients with noncardioembolic moderate or moderate-to-severe ischemic stroke who received ticagrelor within the first 24 h after ischemic stroke had better clinical outcomes based on recurrent stroke rates and unfavorable modified Rankin Scale (mRS) rates compared with those who received clopidogrel. There were no significant variations between ticagrelor and clopidogrel regarding hemorrhagic and non-hemorrhagic complications.
Registration: ClinicalTrials.gov identifier number NCT05553613.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Funding: Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Conflict of Interest: Sherihan Rezk ahmed, Nevine El Nahas, Mohamed Fouad elsayed Khalil, Ahmed elbassiouny, Mohamed Ahmed Almoataz, Tarek youssif omar, Ahmed Mohamed ali Daabis, Hossam Mohamed Refat, Ahmed ahmed Mohamed kamal ebied, Asmaa Mohammed Hassan, Diaa Mostafa Atiaa Mohamed, Mohamed Ismaiel, and Mohamed G. Zeinhom declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript. Authors’ Contributions: Dr Zeinhom and Dr Ahmed were the principal investigators who collected data and Dr. Zeinhom, Dr. Ahmed, Prof. El Nahas, and Prof. Elbassiouny were the main supervisors of the study. Dr. Almoataz, Dr. Omar, Dr. Daabis, Dr. Refat, Dr. Ebied, Dr. Hassan, Dr. Mohamed, Dr. Ismaiel, Dr. Zeinhom, and Dr. Khalil shared in the study plan revision and supervision, while Dr Zeinhom, Dr. Refat, Dr. Ebied, and Dr. Ahmed shared in manuscript writing. All Authors revised the manuscript. Data Availability Statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Guarantor: Mohamed G. Zeinhom is the guarantor of the study. Ethics Approval: Our study had the approval of the ethical committee of Kafr El-sheikh University, and the ethical reference number is (MKSU:50-9-6). Consent to Participate: Written informed consent was obtained from all subjects before enrolment in the study. Consent for Publication: Not applicable. Code Availability: Not applicable.
Figures
References
-
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-110. - PubMed
-
- Ono K, Kurohara K, Yoshihara M, Shimamoto Y, Yamaguchi M. Agranulocytosis caused by ticlopidine and its mechanism. Am J Hematol. 1991;37(4):239–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical