New preclinical biomarkers for prion diseases in the cerebrospinal fluid proteome revealed by mass spectrometry
- PMID: 39520708
- PMCID: PMC11552261
- DOI: 10.1080/01652176.2024.2424837
New preclinical biomarkers for prion diseases in the cerebrospinal fluid proteome revealed by mass spectrometry
Abstract
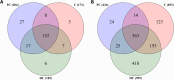
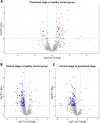
Current diagnostic methods for prion diseases only work in late stages of the disease when neurodegeneration is irreversible. Therefore, biomarkers that can detect the disease before the onset of clinical symptoms are necessary. High-throughput discovery proteomics is of great interest in the search for such molecules. Here we used mass spectrometry to analyse the cerebrospinal fluid proteome in an animal prion disease: preclinical and clinical sheep affected with natural scrapie, and healthy sheep. Interestingly, we found 46 proteins in the preclinical stage that were significantly altered (p < 0.01) compared to healthy sheep, mainly associated with biological processes such as stress and inflammatory responses. Five of them were selected for validation by enzyme-like immunosorbent assay: synaptotagmin binding, cytoplasmic RNA interacting protein (SYNCRIP), involved in nucleic acid metabolism; phospholipase D3 (PLD3) and cathepsin D (CTSD), both related to lysosomal apoptosis; complement component 4 (C4), an element of the classical immune response; and osteopontin (SPP1), a proinflammatory cytokine. These proteins significantly increased in the preclinical stage and maintained their levels in the clinical phase, except for CTSD, whose concentration returned to basal levels in the clinical group. Further research is ongoing to explore their potential as preclinical biomarkers of prion diseases.
Keywords: Prion; biomarkers; cerebrospinal fluid; diagnosis; mass spectrometry; neurodegenerative diseases; preclinical; scrapie.
Conflict of interest statement
No potential competing interest was reported by the authors.
Figures





References
-
- Anjo, S.I., Santa, C., Manadas, B.. 2019. SWATH Mass Spectrometry applied to cerebrospinal fluid differential proteomics: establishment of a sample-specific method. In: Santamaría E, Fernández-Irigoyen J, editors. Cerebrospinal fluid (CSF) proteomics. Methods and protocols. Springer: Methods in Molecular Biology; p. 169–189. doi: 10.1007/978-1-4939-9706-0_11. - DOI - PubMed
-
- Blanc V, Navaratnam N, Henderson JO, Anant S, Kennedy S, Jarmuz A, Scott J, Davidson NO.. 2001. Identification of GRY-RBP as an apolipoprotein B RNA-binding protein that interacts with both apobec-1 and apobec-1 complementation factor to modulate C to U editing. J Biol Chem. 276(13):10272–10283. doi: 10.1074/jbc.M006435200. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
