Mitochondrial bioenergetics deficiency in cisd-1 mutants is linked to AMPK-mediated lipid metabolism
- PMID: 39521176
- PMCID: PMC12319338
- DOI: 10.1016/j.bj.2024.100806
Mitochondrial bioenergetics deficiency in cisd-1 mutants is linked to AMPK-mediated lipid metabolism
Abstract
Background: CISD-1 is a mitochondrial iron-sulfate [2Fe-2S] protein known to be associated with various human diseases, including cancer and diabetes. Previously, we demonstrated that CISD-1 deficiency in worms lowers glucose and ATP levels. In this study, we further explored how worms compensate for lower ATP levels by analyzing changes in cytoplasmic and mitochondrial iron content, AMPK activities, and total lipid profiles.
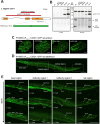
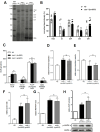
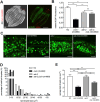
Materials and methods: Expression levels of CISD-1 and CISD-1GFP fusion proteins in wild-type worms (N2), cisd-1-deletion mutants (tm4993 and syb923) and GFP insertion transgenic worms (PHX953 and SJL40) were examined by Western blot. Fluorescence microscopy analyzed CISD-1GFP pattern in PHX953 embryos and adults, and lipid droplet sizes in N2, cisd-1, aak-2 and aak-2;cisd-1 worms. Total and mitochondrial iron content, electron transport complex profiles, and AMPK activity were investigated in tm4993 and syb923 mutants. mRNA levels of mitochondrial β-oxidation genes, acs-2, cpt-5, and ech-1, were quantified by RT-qPCR in various genetic worm strains. Lipidomic analyses were performed in N2 and cisd-1(tm4993) worms.
Results: Defects in cisd-1 lead to an imbalance in iron transport and cause proton leak, resulting in lower ATP production by interrupting the mitochondrial electron transport chain. We identified a signaling pathway that links ATP deficiency-induced AMPK (AMP activated protein kinase) activation to the expression of genes that facilitate lipolysis via β-oxidation.
Conclusion: Our data provide a functional coordination between CISD-1 and AMPK constitutes a mitochondrial bioenergetics quality control mechanism that provides compensatory energy resources.
Keywords: AMPK; C. elegans; Iron-sulfate containing protein; Lipid droplet size; Lipidomic; Mitochondria outer membrane protein.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Moreno-Navarrete JM, Moreno M, Ortega F, Sabater M, Xifra G, Ricart W, et al. CISD1 in association with obesity-associated dysfunctional adipogenesis in human visceral adipose tissue. Obesity. 2016;24(1):139–147. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

