Pim1 inactivating induces RUNX3 upregulation that improves/alleviates airway inflammation and mucus hypersecretion in vitro and in vivo
- PMID: 39521608
- PMCID: PMC11552021
- DOI: 10.1136/bmjresp-2023-002066
Pim1 inactivating induces RUNX3 upregulation that improves/alleviates airway inflammation and mucus hypersecretion in vitro and in vivo
Abstract
Purpose: Our research aimed to evaluate whether proto-oncogene serine/threonine-protein kinase Pim-1 (Pim1) inactivation could attenuate asthma by promoting runt-related transcription factor 3 (Runx3) expression and explore the underlying molecular mechanism.
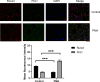
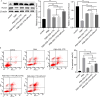
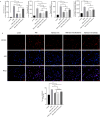
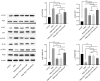
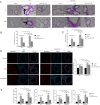
Method: Phorbol 12-myristate 13-acetate (PMA, 50 nM) was used to induce inflammation in BEAS-2B human airway epithelial cells. ELISA and immunofluorescence double staining confirmed inflammation modelling and differential expression of Pim1 and Runx3. Pim1 inhibitor (SGI-1776) and Runx3 siRNA (siRunx3) were used in this study. Apoptosis, inflammation, MUC5AC protein expression, Pim1 kinase and Runx3 protein expression, and PI3K/AKT/nuclear factor-κB (NF-κB) pathway-associated protein expression were also assessed by flow cytometry, immunofluorescence and western blot. The effects of Pim1 inactivation on airway inflammation, pathological injury and mucus secretion in wild-type and Runx3 knockout mice were observed by in vivo experiments.
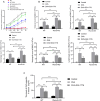
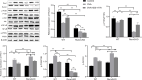
Results: The results of the in vitro experiments showed that PMA stimulation causes BEAS-2B cell apoptosis and promotes the MUC5AC expression. In addition, PMA stimulation activated the PI3K/AKT/NF-κB pathway. SGI-1776 treatment partially reversed these effects, whereas siRunx3 attenuated the effects of SGI-1776 on PMA-stimulated BEAS-2B cells. In vivo experiments showed that in Runx3-KO asthmatic mice, inhibition of Pim1 kinase had less effect on airway inflammation, pathological injury and mucus secretion. Meanwhile, Pim1 kinase expression was higher in Runx3-KO asthmatic mice than in wild-type asthmatic mice. Furthermore, inhibition of Pim1 kinase inhibited activation of the PI3K/AKT/NF-κB pathway, whereas these effects were attenuated in Runx3-KO mice.
Conclusion: Our results suggest that Pim1 inactivation can ameliorate airway inflammation and mucus hypersecretion through upregulation of Runx3 and the effect could be mediated through modulation of the PI3K/AKT/NF-κB pathway.
Keywords: Airway Epithelium; Asthma.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Sheehan WJ, Rangsithienchai PA, Wood RA, et al. Pest and allergen exposure and abatement in inner-city asthma: a work group report of the American Academy of Allergy, Asthma & Immunology Indoor Allergy/Air Pollution Committee. J Allergy Clin Immunol. 2010;125:575–81. doi: 10.1016/j.jaci.2010.01.023. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
