Upregulated let-7 expression in the follicular fluid of patients with endometriomas leads to dysfunction of granulosa cells through targeting of IGF1R
- PMID: 39521729
- PMCID: PMC11700901
- DOI: 10.1093/humrep/deae247
Upregulated let-7 expression in the follicular fluid of patients with endometriomas leads to dysfunction of granulosa cells through targeting of IGF1R
Abstract
Study question: What molecular mechanisms underlie the decline in ovarian reserve as the number and quality of oocytes decrease in patients with ovarian endometriomas (OEM)?
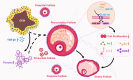
Summary answer: Elevated expression of the let-7 micro(mi)RNAs in the follicular microenvironment of OEM-affected ovaries targets the expression of type 1 insulin-like growth factor receptor (IGF1R) in granulosa cell (GC) and disrupts their proliferation, steroid hormone secretion levels, adenosine triphosphate (ATP) energy metabolism, and reactive oxygen species (ROS) oxidative stress levels.
What is known already: Patients with OEM exhibit diminished ovarian reserve, characterized by reduced oocyte quantity and quality. Fibrotic changes in the ovarian tissue surrounding the OEM create a disruptive microenvironment for follicular growth and development.
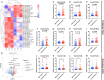
Study design, size, duration: This is a cross-sectional study aimed to elucidate the molecular mechanisms underlying the impact of OEM on follicular development. Initially, miRNA expression profiles in follicular fluid (FF) samples were sequenced from patients with infertility related to OEM (N = 3) and male factor (MF) infertility (N = 3), with the latter serving as the control group. Differentially expressed miRNAs were validated in additional samples from each group (N = 55 in OEM group and N = 45 in MF group) to confirm candidate miRNAs. The study also investigated indicators associated with GCs dysfunction in vitro on rat GCs. Subsequently, rat models of OEM were established through endometrial allogeneic transplantation, and fertility experiments were conducted to assess the let-7/IGF1R axis response to OEM in vivo. Patient samples were collected between May 2018 and April 2019, and the mechanistic study was conducted over the subsequent three years.
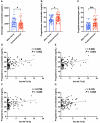
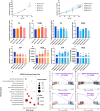
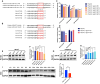
Participants/materials, setting, methods: FF and GC samples were obtained from infertile patients undergoing IVF treatment for OEM and MF related infertility. miRNA expression profiles in FF samples were analyzed using second-generation high-throughput sequencing technology, and candidate miRNAs were validated through quantitative PCR (qPCR). In the in vitro experiments conducted with rat GCs, cell proliferation was assessed using the CCK-8 assay, while steroid hormone concentrations were measured using chemiluminescence. ATP content was determined with an ATP assay kit, and levels of ROS were quantified using flow cytometry. A dual luciferase reporter gene assay was employed to identify the target gene of let-7 based on the construction of a IGF1R reporter gene plasmid using 293T cells. Western blotting was utilized to evaluate the expression of IGF1R in GCs, as well as its downstream proteins, and changes in signaling pathways following let-7 agomir/antagomir transfection and/or Igf1r silencing. In the in vivo OEM rat models, alterations in ovarian structure and cyst morphology were observed using hematoxylin and eosin staining. The expressions of let-7 and Igf1r in GCs were evaluated through qPCR, while variations in IGF1R expression were investigated with immunohistochemistry.
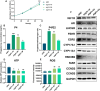
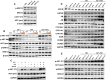
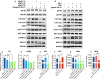
Main results and the role of chance: The cohort of patients with ovarian OEM in this study exhibited significantly decreased antral follicle counts, oocyte retrieval numbers, and normal fertilization rates compared to the control group with MF. The expression of the let-7 miRNA family was markedly upregulated in the FF and GCs of OEM patients. Transfection of rat GCs with let-7 agonists diminished the functions of GCs, including disrupted cell proliferation, mitochondrial oxidative phosphorylation, and steroid hormone secretion, while transfection of rat GCs with let-7 antagonists caused the opposite effects. Luciferase reporter gene experiments confirmed that let-7 complementarily bound to the 3'-untranslated regions of IGF1R. Stimulation of let-7 expression in rat GCs led to a significant decrease in IGF1R expression, while inhibition of let-7 increased IGF1R expression. The expression of IGF1R in the GCs of OEM patients was also significantly reduced compared to MF patients. Silencing of Igf1r led to the dysfunction of GCs, similar to the effects of let-7 agonization, as demonstrated by the downregulation of key proteins involved in cell proliferation (CCND2 and CCND3) and oestradiol synthesis, as well as an increase in progesterone synthesis (StAR), while implicating the PI3K-Akt and MAPK signaling pathways. The antagonistic effect of let-7 on GCs was ineffective when Igf1r was silenced. Conversely, the agonistic effect of let-7 on GCs could be reversed by stimulation with the IGF1R ligand IGF-1. These findings suggested that let-7 regulated the proliferation, differentiation, and ATP synthesis of GCs through targeting IGF1R. The OEM rat model demonstrated alterations in ovarian morphology and structure, along with reduced fertility. Let-7 expression was significantly upregulated in GCs of OEM rats compared to normal rats, while Igf1r and IGF1R expression in pre-ovulatory follicular GCs were notably downregulated, supporting the notion that elevated let-7 expression in the follicular microenvironment of OEM inhibited IGF1R, leading to abnormal GC function and impacting fertility at the molecular level.
Large scale data: N/A.
Limitations, reasons for caution: The synthesis and secretion mechanisms of steroid hormones are intricate and complex. Some enzymes that regulate oestrogen synthesis also play a role in progesterone synthesis. Moreover, certain receptors can respond to multiple hormone signals. Therefore, in this study, the expression patterns of key enzymes such as CYP17A, CYP11A1, HSD3B2, StAR, and receptors including AR, LHCGR, FSHR, ESR2, might be influenced by various factors and might not demonstrate complete consistency.
Wider implications of the findings: Future research will concentrate on investigating the potential impact of ovarian stromal cells on the external microenvironment of follicle growth. Additionally, screening for small molecule drugs that target let-7 and IGF1R actions can be conducted to intervene and modify the ovarian microenvironment, ultimately enhancing ovarian function.
Study funding/competing interest(s): This study received funding from the National Natural Science Foundation of China (grant number 82301851 to L.B.S., grant numbers U23A20403 and U20A20349 to S.Y.Z., and grant number 82371637 to Y.D.D.) and the Natural Science Foundation of Zhejiang Province (grant LTGY23H040010 to F.Z.). The authors have no conflicts of interest to declare.
Keywords: IGF1R; endometriosis; female infertility; follicular fluid; granulosa cells; let-7; ovarian endometriomas (OEM).
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 2005;122:553–563. - PubMed
-
- Busacca M, Vignali M. Ovarian endometriosis: from pathogenesis to surgical treatment. Curr Opin Obstet Gynecol 2003;15:321–326. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

