In situ editing of tumour cell membranes induces aggregation and capture of PD-L1 membrane proteins for enhanced cancer immunotherapy
- PMID: 39521768
- PMCID: PMC11550832
- DOI: 10.1038/s41467-024-54081-9
In situ editing of tumour cell membranes induces aggregation and capture of PD-L1 membrane proteins for enhanced cancer immunotherapy
Abstract
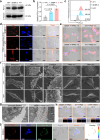
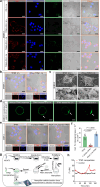
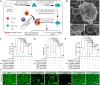
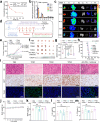
Immune checkpoint blockade (ICB) therapy has emerged as a new therapeutic paradigm for a variety of advanced cancers, but wide clinical application is hindered by low response rate. Here we use a peptide-based, biomimetic, self-assembly strategy to generate a nanoparticle, TPM1, for binding PD-L1 on tumour cell surface. Upon binding with PD-L1, TPM1 transforms into fibrillar networks in situ to facilitate the aggregation of both bound and unbound PD-L1, thereby resulting in the blockade of the PD-1/PD-L1 pathway. Characterizations of TPM1 manifest a prolonged retention in tumour ( > 7 days) and anti-cancer effects associated with reinvigorating CD8+ T cells in multiple mice tumour models. Our results thus hint TPM1 as a potential strategy for enhancing the ICB efficacy.
© 2024. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

