Enhancing anti-EGFRvIII CAR T cell therapy against glioblastoma with a paracrine SIRPγ-derived CD47 blocker
- PMID: 39521782
- PMCID: PMC11550474
- DOI: 10.1038/s41467-024-54129-w
Enhancing anti-EGFRvIII CAR T cell therapy against glioblastoma with a paracrine SIRPγ-derived CD47 blocker
Abstract
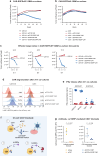
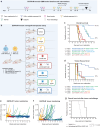
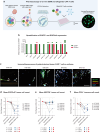
A significant challenge for chimeric antigen receptor (CAR) T cell therapy against glioblastoma (GBM) is its immunosuppressive microenvironment, which is densely populated by protumoral glioma-associated microglia and macrophages (GAMs). Myeloid immune checkpoint therapy targeting the CD47-signal regulatory protein alpha (SIRPα) axis induces GAM phagocytic function, but CD47 blockade monotherapy is associated with toxicity and low bioavailability in solid tumors. In this work, we engineer a CAR T cell against epidermal growth factor receptor variant III (EGFRvIII), constitutively secreting a signal regulatory protein gamma-related protein (SGRP) with high affinity to CD47. Anti-EGFRvIII-SGRP CAR T cells eradicate orthotopic EGFRvIII-mosaic GBM in vivo, promoting GAM-mediated tumor cell phagocytosis. In a subcutaneous CD19+ lymphoma mouse model, anti-CD19-SGRP CAR T cell therapy is superior to conventional anti-CD19 CAR T. Thus, combination of CAR and SGRP eliminates bystander tumor cells in a manner that could overcome main mechanisms of CAR T cell therapy resistance, including immune suppression and antigen escape.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: G.H. has equity in and is a co-founder of Incephalo Inc. H.L. is a member of the Scientific Advisory Board of GlycoEra and InterVenn and has received research support from Palleon Pharmaceuticals and consulting fees from Alector. Patent disclosure for the work described in this study: Chimeric antigen receptor constructs and uses thereof. International Patent Application No. PCT/EP2024/074131 on 30 August 2024. Assignee: the trustees of the University of Basel. Inventors: G.H., T.A.M. The remaining authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
- PP00P3_176974/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 310030_215237/1/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- PP00P3_176974/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- KFS-5763-02-2023/Krebsliga Schweiz (Ligue Suisse Contre le Cancer)
- KFS-4382-02-2018/Krebsliga Schweiz (Ligue Suisse Contre le Cancer)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

