VEGF-C propagates 'onward' colorectal cancer metastasis from liver to lung
- PMID: 39521880
- PMCID: PMC11724081
- DOI: 10.1038/s41416-024-02892-4
VEGF-C propagates 'onward' colorectal cancer metastasis from liver to lung
Abstract
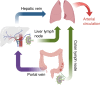
Background: The formation of lung metastasis as part of the progression of colon cancer is a poorly understood process. Theoretically, liver metastases could seed lung metastases.
Methods: To assess the contribution of the liver lymphatic vasculature to metastatic spread to the lungs, we generated murine liver-metastasis-derived organoids overexpressing vascular endothelial growth factor (VEGF)-C. The organoids were reimplanted into the mouse liver for tumour generation and onward metastasis.
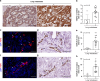
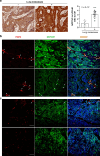
Results: Liver metastases from patients with concomitant lung metastases showed higher expression of VEGF-C, lymphatic vessel hyperplasia, and tumour cell invasion into lymphatic vessels when compared to those without lung metastases. Reimplantation of VEGF-C overexpressing organoids into the mouse liver showed that VEGF-C caused peritumoral lymphatic vessel hyperplasia, lymphatic tumour cell invasion, and lung metastasis formation. This change in metastatic organotropism was accompanied by reduced expression of WNT-driven adult stem cell markers, and increased expression of fetal stem cell markers and NOTCH pathway genes. Further NOTCH pathway inhibition with γ-secretase inhibitor (DAPT) in vivo results in a slight reduction in lung metastases and a decrease in lymphatic hyperplasia and invasion in VEGF-C-overexpressing tumours.
Conclusion: Collectively, these data indicate that VEGF-C can drive onward metastasis from the liver to the lung and suggest that targeting VEGF-C/NOTCH pathways may impair the progression of colorectal cancer.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All experiments with human tissues were approved by the Biobank Research Ethics Committee of the University Medical Centre Utrecht (Utrecht, the Netherlands). Written informed consent from the donors for the research use of tissue in this study was obtained prior to the acquisition of the specimen. The study work protocol (614-1-17) involving laboratory animals was approved by Utrecht University’s Animal Welfare Body, the Animal Ethics Committee and licensed by the Central Authority for Scientific Procedures on Animals (license numbers AVD115002016614, AVD11500202115055). All experiments were conducted in accordance with the Dutch Experiments on Animals Act, in line with European Directive 2010/63/EU and by licensed personnel. Consent for publication: Not applicable.
Figures






References
-
- Punt CJA, Koopman M, Vermeulen L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat Rev Clin Oncol. 2016;14:235–46. - PubMed
-
- Petrowsky H, Fritsch R, Guckenberger M, De Oliveira ML, Dutkowski P, Clavien P-A. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat Rev Gastroenterol Hepatol. 2020;17:755–72. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

