Oncolytic immunotherapy with nivolumab in muscle-invasive bladder cancer: a phase 1b trial
- PMID: 39521884
- PMCID: PMC12302721
- DOI: 10.1038/s41591-024-03324-9
Oncolytic immunotherapy with nivolumab in muscle-invasive bladder cancer: a phase 1b trial
Abstract
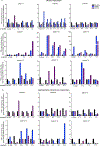
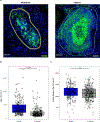
There is a critical unmet need for safe and efficacious neoadjuvant treatment for cisplatin-ineligible patients with muscle-invasive bladder cancer. Here we launched a phase 1b study using the combination of intravesical cretostimogene grenadenorepvec (oncolytic serotype 5 adenovirus encoding granulocyte-macrophage colony-stimulating factor) with systemic nivolumab in cisplatin-ineligible patients with cT2-4aN0-1M0 muscle-invasive bladder cancer. The primary objective was to measure safety, and the secondary objective was to assess the anti-tumor efficacy as measured by pathologic complete response along with 1-year recurrence-free survival. No dose-limiting toxicity was encountered in 21 patients enrolled and treated. Combination treatment achieved a pathologic complete response rate of 42.1% and a 1-year recurrence-free survival rate of 70.4%. Pathologic response was associated with baseline free E2F activity and tumor mutational burden but not PD-L1 status. Although T cell infiltration was broadly induced after intravesical oncolytic immunotherapy, the formation, enlargement and maturation of tertiary lymphoid structures was specifically associated with complete response, supporting the importance of coordinated humoral and cellular immune responses. Together, these results highlight the potential of this combination regimen to enhance therapeutic efficacy in cisplatin-ineligible patients with muscle-invasive bladder cancer, warranting additional study as a neoadjuvant therapeutic option. ClinicalTrials.gov identifier: NCT04610671 .
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests: R.L. reports research support from Predicine, Veracyte, CG Oncology, Valar Labs, Merck and Janssen; clinical trial protocol committee participation with CG Oncology, Merck and Janssen; and is scientific advisor/consultant for Bristol Myers Quibb, Merck, Fergene, Arquer Diagnostics, Urogen Pharma, Lucence, CG Oncology, Janssen, Thericon, Iconovir, ImmunityBio and Pfizer. W.J.S. reports consultant work for Pacific Edge and Urogen Pharma. P.E.S. reports being the Vice Chair of the NCCN guidelines committee for bladder and penile cancer. J.Z. reports consultant work for Sanofi, AstraZeneca, Dendreon, Seagen, Bayer and Pfizer. G.D.G. reports consultant work for MyCareGorithm and stock options in Lantheus. J.M.B. reports consultant work for CG Oncology and Kalivir Immunotherapeutics and being a shareholder of CG Oncology and Kalivir Immunotherapeutics. C.P.N.D. reports consultant work for AstraZeneca and CG Oncology and intellectual property ownership related to the use of genetic alterations as a predictive biomarker for response to nadofaragene firadenovec. R.K.J. reports consultant work for AVEO, Bristol Myers Squibb, Sanofi, EMD Serono, Gilead Sciences, IDEOlogy Pfizer and Seattle Genetics/Astellas; speaker’s bureau for Gilead Sciences, Seagen and Seattle Genetics/Astellas; research funding from Bristol Myers Squibb, Gilead Sciences and the National Cancer Institute; and honoraria from FLASCO, Curio Science, DAVA Oncology, NCCN/Pfizer and OncLive/MJH Life Sciences. J.J.M. reports membership on the CG Oncology Board of Directors; being the Associate Center Director at Moffitt Cancer Center; ownership interest in Aleta Biotherapeutics, CG Oncology, Turnstone Biologics, Ankyra Therapeutics and AffyImmune Therapeutics; and consultant work for ONCoPEP, CG Oncology, Turnstone Biologics, Vault Pharma, Ankyra Therapeutics, AffyImmune Therapeutics, UbiVac, Vycellix and Aleta Biotherapeutics. J.R.C.-G. reports consultant work for Anixa Biosciences and Alloy Therapeutics; stock options in Compass Therapeutics, Anixa Biosciences and Alloy Therapeutics; patent licensed by Anixa Biosciences; intellectual property filed with Compass Therapeutics; and being the co-founder of Cellepus Therapeutics, a CAR T cell company. The other authors declare no competing interests.
Figures
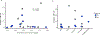

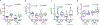






Comment in
-
Neoadjuvant therapy in MIBC.Nat Rev Urol. 2025 Jan;22(1):5. doi: 10.1038/s41585-024-00989-2. Nat Rev Urol. 2025. PMID: 39663416 No abstract available.
References
-
- Stein JP et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 19, 666–675 (2001). - PubMed
-
- Grossman HB et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 349, 859–866 (2003). - PubMed
-
- Flaig TW et al. NCCN Guidelines® Insights: Bladder Cancer, Version 2.2022. J. Natl Compr. Canc. Netw. 20, 866–878 (2022). - PubMed
-
- Galsky MD et al. Treatment of patients with metastatic urothelial cancer ‘unfit’ for cisplatin-based chemotherapy. J. Clin. Oncol. 29, 2432–2438 (2011). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

