Identification and functional analysis of novel SPTB and ANK1 mutations in hereditary spherocytosis patients
- PMID: 39521890
- PMCID: PMC11550412
- DOI: 10.1038/s41598-024-78622-w
Identification and functional analysis of novel SPTB and ANK1 mutations in hereditary spherocytosis patients
Abstract
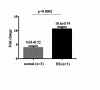
Hereditary spherocytosis (HS) is the most prevalent form of congenital hemolytic anemia, being caused by genetic mutations in genes encoding red blood cell cytoskeletal proteins. Mutations in the ANK1 and SPTB genes are the most common causes of HS.; however, pathogenicity analyses of these mutations remain limited. This study identified three novel heterozygous mutations in 3 HS patients: c.1994 C > A in ANK1, c.5692 C > T, and c.3823delG in SPTB by whole-exome sequencing (WES) and validated by Sanger sequencing. To investigate the functional consequences of these mutations, we studied their pathogenicity using in vitro culture erythroblast derived from CD34 + stem cells. All three mutations lead to the generation of a premature stop codon. Real-time PCR assay revealed that the two SPTB mutations resulted in reduced SPTB mRNA expression, suggesting a potential role for the nonsense-mediated mRNA degradation pathway. For the ANK1 mutation, gene expression was not reduced but was predicted to produce a truncated version of the ANK1 protein. Flow cytometry analysis of red blood cell-derived microparticles (MPs) revealed that HS patients had higher MP levels compared to normal subjects. This study contributes to the current understanding of the molecular mechanisms underlying mutations in the ANK1 and SPTB genes in HS.
Keywords: ANK1; DNA sequencing; Hereditary spherocytosis; Mutation; NGS; SPTB.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

