Precision-cut liver slices as an ex vivo model to assess impaired hepatic glucose production
- PMID: 39521914
- PMCID: PMC11550398
- DOI: 10.1038/s42003-024-07070-z
Precision-cut liver slices as an ex vivo model to assess impaired hepatic glucose production
Abstract
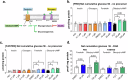
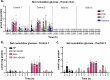
Fasting hypoglycemia is a severe and incompletely understood symptom of various inborn errors of metabolism (IEM). Precision-cut liver slices (PCLS) represent a promising model for studying glucose production ex vivo. This study quantified the net glucose production of human and murine PCLS in the presence of different gluconeogenic precursors. Dihydroxyacetone-supplemented slices from the fed mice yielded the highest rate, further stimulated by forskolin and dibutyryl-cAMP. Moreover, using 13C isotope tracing, we assessed the contribution of glycogenolysis and gluconeogenesis to net glucose production over time. Pharmacological inhibition of the glucose 6-phosphate transporter SLC37A4 markedly reduced net glucose production and increased lactate secretion and glycogen storage, while glucose production was completely abolished in PCLS from glycogen storage disease type Ia and Ib patients. In conclusion, this study identifies PCLS as an effective ex vivo model to study hepatic glucose production and opens opportunities for its future application in IEM research and beyond.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
- 812616/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
- 812616/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
- 812616/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
- 812616/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
LinkOut - more resources
Full Text Sources

