Changes in lipid abundance are associated with disease progression and treatment response in chronic Trypanosoma cruzi infection
- PMID: 39521974
- PMCID: PMC11549750
- DOI: 10.1186/s13071-024-06548-3
Changes in lipid abundance are associated with disease progression and treatment response in chronic Trypanosoma cruzi infection
Abstract
Background: Chagas disease, caused by the parasite Trypanosoma cruzi, is a zoonosis that affects more than seven million people. Current limitations on the diagnosis of the disease hinder the prognosis of patients and the evaluation of treatment efficacy, slowing the development of new therapeutic options. The infection is known to disrupt several host metabolic pathways, providing an opportunity for the identification of biomarkers.
Methods: The metabolomic and lipidomic profiles of a cohort of symptomatic and asymptomatic patients with T. cruzi infection and a group of uninfected controls were analysed using liquid chromatography/mass spectrometry. Differences among all groups and changes before and after receiving anti-parasitic treatment across those with T. cruzi infection were explored.
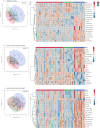
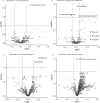
Results: Three lipids were found to differentiate between symptomatic and asymptomatic participants: 10-hydroxydecanoic acid and phosphatidylethanolamines PE(18:0/20:4) and PE(18:1/20:4). Additionally, sphinganine, 4-hydroxysphinganine, hexadecasphinganine, and other sphingolipids showed post-treatment abundance similar to that in non-infected controls.
Conclusions: These molecules hold promise as potentially useful biomarkers for monitoring disease progression and treatment response in patients with chronic T. cruzi infection.
Keywords: Trypanosoma cruzi; Hydroxydecanoic acid; Chagas disease; Lipidomics; Metabolomics; Phosphatidylethanolamine; Sphingolipids; Treatment response.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Pérez-Molina JA, Molina I. Chagas disease. Lancet. 2018;391:82–94. - PubMed
-
- Alonso-Padilla J, López MC, Esteva M, Zrein M, Casellas A, Gómez I, et al. Serological reactivity against T. cruzi-derived antigens: evaluation of their suitability for the assessment of response to treatment in chronic Chagas disease. Acta Trop. 2021;221:105990. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

