Pilot evaluation on an adapted tele-behavioral activation to increase physical activity in persons with depression: a single-arm pilot study
- PMID: 39522018
- PMCID: PMC11549759
- DOI: 10.1186/s40359-024-02053-5
Pilot evaluation on an adapted tele-behavioral activation to increase physical activity in persons with depression: a single-arm pilot study
Abstract
Background: Physical activity has the potential to improve physical and mental health outcomes of persons with depression. However, feasible and acceptable strategies to integrate physical activity interventions into real-world settings are needed.
Objective: To assess the feasibility and acceptability of a manualized Behavioral Activation intervention aimed to increase physical activity in persons with depression (defined as a PHQ-9 score ≥ 10).
Methods: A single-arm pilot study was conducted. The intervention consisted of 8 tele-therapy sessions delivered over a 10-week period. Measures of feasibility included screening, enrollment, intervention adherence, outcome data availability, and intervention fidelity. Acceptability was assessed with a post-intervention survey and qualitatively through focus groups and interviews. Preliminary efficacy of the intervention was assessed by evaluating pre-to-post changes in physical activity and depressive symptoms.
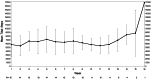
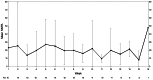
Results: All feasibility metrics exceeded predetermined feasibility goal metrics with the exception of Fitbit wear and screening rate, which was due to a greater than anticipated enrollment rate. Participants (n = 15) reported perceived benefits from the intervention and convenience in attending tele-therapy sessions. Depressive symptoms, as measured by the PHQ-9 improved (16.8 at enrollment to 10.1 post intervention, Cohen's d = 1.13). Self-reported moderate-to-vigorous physical activity (MVPA) increased from 22.0 min/week at baseline to 36.67 min/week post-intervention (d = 0.58). Physical activity as measured by the Fitbit showed little change (daily step 5543.29 during Week 1 to 6177.48 during Week 10, (d = 0.14); MVPA 21.23 min/week during Week 1 to 19.22 at Week 10 (d = 0.0.06).
Conclusions: Results of the pilot study suggest the intervention is feasible to deliver and acceptable to participants. Preliminary results suggest the intervention may be effective in improving depressive symptoms and increasing self-reported physical activity.
Trial registration: ClinicalTrials.gov NCT04990401, Registered July 21, 2021.
Keywords: Behavioral activation; Depression; Exercise; Physical activity.
© 2024. The Author(s).
Conflict of interest statement
Joseph Trombello is a shareholder in Merck, unrelated to the current project. Within the past 36 months, Dr. Trivedi has provided consulting services to ACADIA Pharmaceuticals, Allergan, Alkermes Inc., Alto Neuroscience Inc, Applied Clinical Intelligence, LLC, Axsome Therapeutics, Biogen MA Inc, Boegringer Ingelheim, Cerebral Inc., Circular Genomics Inc., Compass Pathfinder Limited, GH Research, GreenLight VitalSign6 Inc, Heading Health, Health Care Global Village, Janssen Pharmaceutical, Jazz Pharmaceutical, Legion Health, Lundbeck Research USA, Merck Sharp & Dohme Corp., Mind Medicine Inc., Myriad Neuroscience, Naki Health Ltd, Navitor, Neurocrine Biosciences Inc., Noema Pharma AG, Orexo US Inc., Otsuka America Pharmaceutical Inc, Perception Neuroscience Holdings, Pharmerit International, Policy Analysis Inc., Praxis Precision Medicines Inc, Rexahn Pharmaceuticals, Inc., SAGE Therapeutics, Signant Health, Sparian Biosciences, Titan Pharmaceuticals, Takeda Pharmaceuticals Inc, WebMD. He has received grant/research funding from NIMH, NIDA, NCATS, American Foundation for Suicide Prevention, Patient-Centered Outcomes Research Institute (PCORI), and Blue Cross Blue Shield of Texas. Additionally, he has received editorial compensation from Engage Health Media, and Oxford University Press. All other authors have no competing interests to report.
Figures
Similar articles
-
Web-Based Intervention Using Behavioral Activation and Physical Activity for Adults With Depression (The eMotion Study): Pilot Randomized Controlled Trial.J Med Internet Res. 2018 Jul 16;20(7):e10112. doi: 10.2196/10112. J Med Internet Res. 2018. PMID: 30012547 Free PMC article. Clinical Trial.
-
Feasibility and Acceptability of a Counseling- and mHealth-Based Physical Activity Intervention for Pregnant Women With Diabetes: The Fit for Two Pilot Study.JMIR Mhealth Uhealth. 2020 Oct 21;8(10):e18915. doi: 10.2196/18915. JMIR Mhealth Uhealth. 2020. PMID: 33084584 Free PMC article.
-
Behavioral health coaching for rural-living older adults with diabetes and depression: an open pilot of the HOPE Study.BMC Geriatr. 2012 Jul 24;12:37. doi: 10.1186/1471-2318-12-37. BMC Geriatr. 2012. PMID: 22828177 Free PMC article. Clinical Trial.
-
Gaming-Based Tele-Exercise Program to Improve Physical Function in Frail Older Adults: Feasibility Randomized Controlled Trial.J Med Internet Res. 2024 Nov 27;26:e56810. doi: 10.2196/56810. J Med Internet Res. 2024. PMID: 39602215 Free PMC article. Clinical Trial.
-
Development and evaluation of a de-escalation training intervention in adult acute and forensic units: the EDITION systematic review and feasibility trial.Health Technol Assess. 2024 Jan;28(3):1-120. doi: 10.3310/FGGW6874. Health Technol Assess. 2024. PMID: 38343036 Free PMC article.
References
-
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155–62. - PubMed
-
- Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Differential mortality rates in major and subthreshold depression: meta-analysis of studies that measured both. Br J Psychiatry. 2013;202(1):22–7. - PubMed
-
- Thase ME, Haight BR, Richard N, Rockett CB, Mitton M, Modell JG, et al. Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials. J Clin Psychiatry. 2005;66(8):974–81. - PubMed
-
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–17. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical