Identifying and optimizing critical process parameters for large-scale manufacturing of iPSC derived insulin-producing β-cells
- PMID: 39522051
- PMCID: PMC11550522
- DOI: 10.1186/s13287-024-03973-0
Identifying and optimizing critical process parameters for large-scale manufacturing of iPSC derived insulin-producing β-cells
Abstract
Background: Type 1 diabetes, an autoimmune disorder leading to the destruction of pancreatic β-cells, requires lifelong insulin therapy. Islet transplantation offers a promising solution but faces challenges such as limited availability and the need for immunosuppression. Induced pluripotent stem cells (iPSCs) provide a potential alternative source of functional β-cells and have the capability for large-scale production. However, current differentiation protocols, predominantly conducted in hybrid or 2D settings, lack scalability and optimal conditions for suspension culture.
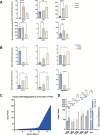
Methods: We examined a range of bioreactor scaleup process parameters and quality target product profiles that might affect the differentiation process. This investigation was conducted using an optimized High Dimensional Design of Experiments (HD-DoE) protocol designed for scalability and implemented in 0.5L (PBS-0.5 Mini) vertical wheel bioreactors.
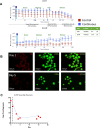
Results: A three stage suspension manufacturing process is developed, transitioning from adherent to suspension culture, with TB2 media supporting iPSC growth during scaling. Stage-wise optimization approaches and extended differentiation times are used to enhance marker expression and maturation of iPSC-derived islet-like clusters. Continuous bioreactor runs were used to study nutrient and growth limitations and impact on differentiation. The continuous bioreactors were compared to a Control media change bioreactor showing metabolic shifts and a more β-cell-like differentiation profile. Cryopreserved aggregates harvested from the runs were recovered and showed maintenance of viability and insulin secretion capacity post-recovery, indicating their potential for storage and future transplantation therapies.
Conclusion: This study demonstrated that stage time increase and limited media replenishing with lactate accumulation can increase the differentiation capacity of insulin producing cells cultured in a large-scale suspension environment.
Keywords: Bioprocess development; Bioreactor; Diabetes; DoE; Human induced pluripotent stem cell; Insulin producing cells; Islets; Optimization; Pancreatic cells; iPSCs; β-cells.
© 2024. The Author(s).
Conflict of interest statement
J.J. is founder of and shareholder of Trailhead Biosystems, Inc., Beachwood, OH, USA. M. B. is a shareholder in Trailhead Biosystems, Inc., Beachwood OH. This work has been filed as US Provisional Application No. application pending.
Figures




References
-
- T1D Index Website. 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

