Thermoregulation and survival during sepsis: insights from the cecal ligation and puncture experimental model
- PMID: 39522078
- PMCID: PMC11551088
- DOI: 10.1186/s40635-024-00687-8
Thermoregulation and survival during sepsis: insights from the cecal ligation and puncture experimental model
Abstract
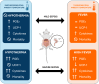
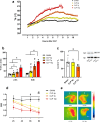
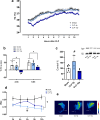
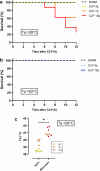
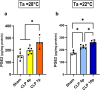
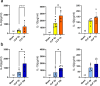
Background: Sepsis remains a major global health concern due to its high prevalence and mortality. Changes in body temperature (Tb), such as hypothermia or fever, are diagnostic indicators and play a crucial role in the pathophysiology of sepsis. This study aims to characterize the thermoregulatory mechanisms during sepsis using the cecal ligation and puncture (CLP) model and explore how sepsis severity and ambient temperature (Ta) influence Tb regulation and mortality. Rats were subjected to mild or severe sepsis by CLP while housed at thermoneutral (28 °C) or subthermoneutral (22 °C) Ta, and their Tb was monitored for 12 h. Blood and hypothalamus were collected for cytokines and prostaglandin E2 (PGE2) analysis.
Results: At 28 °C, febrile response magnitude correlated with sepsis severity and inflammatory response, with tail vasoconstriction as the primary heat retention mechanism. At 22 °C, Tb was maintained during mild sepsis but dropped during severe sepsis, linked to reduced UCP1 expression in brown adipose tissue and less effective vasoconstriction. Despite differences in thermoregulatory responses, both Ta conditions induced a persistent inflammatory response and increased hypothalamic PGE2 production. Notably, mortality in severe sepsis was significantly higher at 28 °C (80%) compared to 22 °C (0%).
Conclusions: Our findings reveal that ambient temperature and the inflammatory burden critically influence thermoregulation and survival during early sepsis. These results emphasize the importance of considering environmental factors in preclinical sepsis studies. Although rodents in experimental settings are often adapted to cold environments, these conditions may not fully translate to human sepsis, where cold adaptation is rare. Thus, researchers should carefully consider these variables when designing experiments and interpreting translational implications.
Keywords: Cytokines; Fever; Hypothalamus; Hypothermia; Mortality; PGE2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Angiotensin-(1-7) improves tail skin heat loss and increases the survival of rats with polymicrobial sepsis.Peptides. 2023 Sep;167:171042. doi: 10.1016/j.peptides.2023.171042. Epub 2023 Jun 12. Peptides. 2023. PMID: 37315714
-
Brain eicosanoids and LPS fever: species and age differences.Prog Brain Res. 1998;115:141-57. doi: 10.1016/s0079-6123(08)62034-8. Prog Brain Res. 1998. PMID: 9632934 Review.
-
Thermoregulation and vasopressin secretion during polymicrobial sepsis.Neuroimmunomodulation. 2009 Jan;16(1):45-53. doi: 10.1159/000179666. Epub 2008 Dec 15. Neuroimmunomodulation. 2009. PMID: 19077445
-
Role of hypothalamic interleukin-1beta in fever induced by cecal ligation and puncture in rats.Am J Physiol. 1998 Sep;275(3):R754-61. doi: 10.1152/ajpregu.1998.275.3.R754. Am J Physiol. 1998. PMID: 9728072
-
Modulation of acyl and des-acyl ghrelin on autonomic and behavioral thermoregulation in various ambient temperatures.J Therm Biol. 2023 Apr;113:103543. doi: 10.1016/j.jtherbio.2023.103543. Epub 2023 Mar 22. J Therm Biol. 2023. PMID: 37055119 Review.
Cited by
-
Largely ignored-but pathogenetically significant: ambient temperature in rodent sepsis models.Intensive Care Med Exp. 2024 Nov 14;12(1):104. doi: 10.1186/s40635-024-00693-w. Intensive Care Med Exp. 2024. PMID: 39542953 Free PMC article. No abstract available.
-
Impact of thermoneutral acclimation on a murine model of polymicrobial peritonitis.PLoS One. 2025 May 30;20(5):e0322855. doi: 10.1371/journal.pone.0322855. eCollection 2025. PLoS One. 2025. PMID: 40445962 Free PMC article.
References
-
- Branco LGS, Soriano RN, Steiner AA (2014) Gaseous mediators in temperature regulation. Compr Physiol. 10.1002/cphy.c130053 - PubMed
-
- Ishiwata T, Hasegawa H, Yasumatsu M et al (2001) The role of preoptic area and anterior hypothalamus and median raphe nucleus on thermoregulatory system in freely moving rats. Neurosci Lett. 10.1016/S0304-3940(01)01865-1 - PubMed
-
- Boulant JA (2000) Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 10.1086/317521 - PubMed
-
- Rand RP, Burton AC, Ing T (1965) The tail of the rat, in temperature regulation and acclimatization. Can J Physiol Pharmacol. 10.1139/y65-025 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

