Alteplase in COVID-19 severe hypoxemic respiratory failure: the TRISTARDS multicenter randomized trial
- PMID: 39522090
- PMCID: PMC11551089
- DOI: 10.1186/s13613-024-01386-z
Alteplase in COVID-19 severe hypoxemic respiratory failure: the TRISTARDS multicenter randomized trial
Abstract
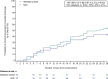
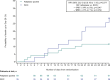
Background: Pulmonary intravascular thrombus formation has been widely observed in patients with respiratory failure, for example, in patients with SARS-CoV-2 infection (COVID-19). The aim of this study was to evaluate the efficacy/safety of alteplase thrombolysis in COVID-19 severe hypoxemic respiratory failure. In this multicenter, open-label study, patients were randomized to receive alteplase (low- or high-dose) over 5 days plus standard of care (SOC), or SOC alone. The primary endpoint was time to clinical improvement (≥ 2-point decrease on WHO Clinical Progression Scale, or hospital discharge) up to Day 28. Secondary endpoints included all-cause mortality at Day 28, treatment failure at Day 28 and change in arterial oxygen partial pressure/fractional inspired oxygen (PaO2/FiO2) ratio at Day 6 versus baseline.
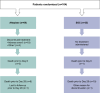
Results: Sixty-nine patients were randomized to alteplase (low- or high-dose) and 35 to SOC; 65% were on high-flow oxygen or non-invasive ventilation at baseline. Median time to clinical improvement was 25 days in the alteplase group and > 28 days (median not reached) in the SOC group. All-cause mortality was 8/69 (12%) versus 10/35 (29%) in the alteplase versus SOC groups, respectively (unadjusted risk difference [RD], - 17% [95% confidence interval (CI) - 34 to 0], p = 0.047; adjusted RD, - 16% [95% CI - 31 to 1], p = 0.058). The PaO2/FiO2 ratio (mean [standard deviation]) increased by + 30 (84) mmHg in the alteplase group and decreased by - 12 (59) mmHg in the SOC group (adjusted mean difference vs. SOC, p = 0.052). Differences were greater in patients receiving high-dose alteplase, and in those not receiving invasive ventilation. Eighteen patients (26.1%) in the alteplase group discontinued treatment due to adverse events. Major bleeding was more frequent with alteplase than with SOC (9 vs. 0 patients); no bleeding was fatal. The study closed early due to insufficient patient recruitment.
Conclusion: Alteplase was not associated with faster clinical recovery from COVID-19 severe hypoxemic respiratory failure. A numerical difference in survival and PaO2/FiO2 ratio was observed, particularly in patients not receiving invasive ventilation. These exploratory findings merit further investigation in larger patient cohorts that are adequately powered to confirm the hypotheses generated in this study regarding the impact of alteplase on treatment outcomes. Trial registration ClinicalTrials.gov: NCT04640194 (November 23, 2020); https://clinicaltrials.gov/study/NCT04640194 (early discontinuation due to insufficient patient recruitment).
Keywords: ARDS; Alteplase; COVID-19; Severe hypoxemic respiratory failure; Thrombolysis.
© 2024. The Author(s).
Conflict of interest statement
Giovanni Landoni reports personal fees from Boehringer Ingelheim for participation on an advisory board. Ferhat Meziani and Jacques Creteur have nothing to declare. Nicolas De Schryver reports personal fees from Trium CC for his role on a data safety monitoring board. Johann Motsch reports payment to his institution by Boehringer Ingelheim for the funding and provision of materials for the TRISTARDS study. Pratima Chowdary declares participation on an advisory board for Boehringer Ingelheim, and receipt of a free drug for a tPA trial in COVID-19 to the Royal Free London NHS Foundation Trust. Ingrid Henrichmoeller, Alain Pagès and Nuala Peters are full-time employees of Boehringer Ingelheim International GmbH. Thierry Danays reports consulting fees from Boehringer Ingelheim paid to TDC. Markus A. Weigand reports grants or contracts from Köhler Chemie, DFG and BMBF, consulting fees from B. Braun, Gilead, Mundipharma and Boehringer Ingelheim, and payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from MSD, Gilead, Shionogi, Pfizer and Beckman Coulter. Dr Weigand is also a patent owner (EP17185036.5 and EP17198330.7), has participated on a data safety monitoring board or advisory board for MSD, Gilead, Shionogi, Biotest, Pfizer, Eumedica, SOBI and Beckman Coulter, is the vice-head of the German Sepsis Society and the scientific advisory council PEG, and is cofounder of Delta Theragnostics.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

