Modulation of connexin 43 in viral infections
- PMID: 39522757
- PMCID: PMC11607658
- DOI: 10.1016/j.tvr.2024.200296
Modulation of connexin 43 in viral infections
Abstract
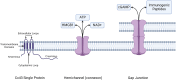
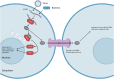
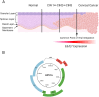
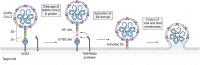
Connexins are essential for intercellular communication through gap junctions and the maintenance of cellular and tissue homeostasis. Connexin 43 (Cx43) is the most ubiquitously expressed connexin. As well as regulating homeostasis, Cx43 hemichannels and gap junctions play important roles in inflammation and the immune response. This, coupled with a range of non-channel functions performed by Cx43 makes it an attractive target for viruses. Recently, several groups have begun to explore the relationship between Cx43 and viral infection, with a diverse array of viruses being found to alter Cx43 hemichannels/gap junctions. Importantly, this includes several small DNA tumour viruses, which may target Cx43 to promote tumorigenesis. This review focuses on the ability of selected RNA/DNA viruses and retroviruses to either positively or negatively regulate Cx43 hemichannels and gap junctions in order to carry out their lifecycles. The role of Cx43 regulation by tumour viruses is also discussed in relation to tumour progression.
Keywords: Connexin 43; Gap junctions; Hemichannels; Human adenovirus type 5; Human immunodeficiency virus; Human papillomavirus; Severe acute respiratory syndrome coronavirus 2.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Gap junction connexins in female reproductive organs: implications for women's reproductive health.Hum Reprod Update. 2015 May-Jun;21(3):340-52. doi: 10.1093/humupd/dmv007. Epub 2015 Feb 9. Hum Reprod Update. 2015. PMID: 25667189
-
Connexin-43 remodelling and arrhythmias: hemichannels as key drivers of cardiac dysfunction.J Physiol. 2025 Aug;603(15):4293-4306. doi: 10.1113/JP288091. Epub 2025 May 5. J Physiol. 2025. PMID: 40320914 Review.
-
Skin disease-associated GJB4 variants differentially influence connexin stability, cell viability and channel function.J Physiol. 2025 Aug;603(15):4383-4407. doi: 10.1113/JP286367. Epub 2025 Jan 16. J Physiol. 2025. PMID: 39817844
-
Phosphorylation-dependent allosteric regulation of Cx43 gap junction inhibitor potency.Biomed Pharmacother. 2024 May;174:116550. doi: 10.1016/j.biopha.2024.116550. Epub 2024 Apr 8. Biomed Pharmacother. 2024. PMID: 38593702
-
Osteocyte connexin hemichannels and prostaglandin E2 release dictate bone marrow mesenchymal stromal cell commitment.Proc Natl Acad Sci U S A. 2025 Feb 18;122(7):e2412144122. doi: 10.1073/pnas.2412144122. Epub 2025 Feb 12. Proc Natl Acad Sci U S A. 2025. PMID: 39937859 Free PMC article.
Cited by
-
Coordination of innate immune responses by connexins.Front Immunol. 2025 May 22;16:1594015. doi: 10.3389/fimmu.2025.1594015. eCollection 2025. Front Immunol. 2025. PMID: 40475783 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

