Potential role of heterologous flavivirus immunity in preventing urban transmission of yellow fever virus
- PMID: 39523371
- PMCID: PMC11551182
- DOI: 10.1038/s41467-024-54146-9
Potential role of heterologous flavivirus immunity in preventing urban transmission of yellow fever virus
Abstract
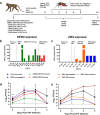
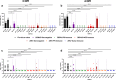
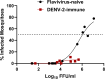
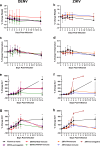
During the recent yellow fever (YF) epidemics in Brazil, human cases were attributed to spillover infections via sylvatic mosquito transmission. Despite YF virus (YFV) transmission in major urban centers with insufficient vaccination coverage and abundant populations of the domestic vector, Aedes aegypti, there was no evidence of human-amplified transmission. Furthermore, the historic absence of YF in Asia, despite abundant Ae. aegypti and an immunologically naive human population, is unexplained. We tested the hypothesis that pre-existing, heterologous flavivirus immunity, specifically from dengue (DENV) and Zika (ZIKV) viruses, limits YFV viremia and transmission by Ae. aegypti. We infected cynomolgus macaques with DENV or ZIKV, then challenged them 6-9 months later with YFV. We then measured viremia and disease and allowed Ae. aegypti mosquitoes to feed during peak macaque viremia. Although prior heterologous immunity had variable effects on disease, DENV and ZIKV immunity consistently suppressed YFV viremia. Despite no statistical difference due to a small sample size, the suppression in viremia led to a significant reduction in Ae. aegypti infection and a lack of transmission potential. These results support the hypothesis that, in DENV- and ZIKV-endemic regions such as South America and Asia, human flavivirus immunity suppresses YFV human amplification potential, reducing the risk of urban outbreaks.
© 2024. The Author(s).
Conflict of interest statement
Figures
Update of
-
Yellow Fever Emergence: Role of Heterologous Flavivirus Immunity in Preventing Urban Transmission.bioRxiv [Preprint]. 2024 Mar 3:2024.03.03.583168. doi: 10.1101/2024.03.03.583168. bioRxiv. 2024. Update in: Nat Commun. 2024 Nov 10;15(1):9728. doi: 10.1038/s41467-024-54146-9. PMID: 38463973 Free PMC article. Updated. Preprint.
References
-
- Monath, T. P. & Barrett, A. D. T. Pathogenesis and pathophysiology of yellow fever. in Advances in Virus Research, Vol. 60 343–395 (Elsevier, 2003). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

