Impact of high-fructose diet and metformin on histomorphological and molecular parameters of reproductive organs and vaginal microbiota of female rat
- PMID: 39523383
- PMCID: PMC11551161
- DOI: 10.1038/s41598-024-76211-5
Impact of high-fructose diet and metformin on histomorphological and molecular parameters of reproductive organs and vaginal microbiota of female rat
Abstract
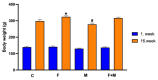
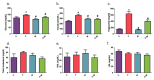
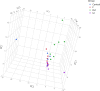
There are limited data on the effects of a high-fructose diet on the female reproductive system. Although metformin has some functional effects on female fertility, its reproductive outcome on high fructose diet-induced metabolic syndrome is unclear. The aim of the present study is to evaluate the impact of a high fructose diet on histomorphological and molecular parameters of the reproductive organs and vaginal microbiota as well as the treatment potential of metformin. Wistar albino rats were used in the study. The metabolic syndrome model was induced by a high-fructose diet in rats for 15 weeks. Metformin was orally administered once a day for the last 6 weeks. The high-fructose diet increased blood glucose, triglycerides, insulin, and ovarian testosterone levels; however, it reduced ovarian aromatase levels and follicle numbers and caused uterine inflammation. The high-fructose diet-induced molecular abnormalities on ovarian tissue were demonstrated by the downregulation of ovarian insulin signaling pathway proteins and dysregulation of ovarian mitogenic and apoptotic pathway proteins. A high-fructose diet caused vaginal dysbiosis, metformin increased probiotic bacteria in the vaginal microbiota. Our results revealed that metformin improves ovarian impairments by modulating hormonal balance, insulin level, mapk, and apoptotic signaling molecules, as well as regulating the vaginal microbiota.
Keywords: High-fructose diet; Metformin; Ovarian tissue; Signaling pathways; Vaginal microbiota.
© 2024. The Author(s).
Conflict of interest statement
Figures




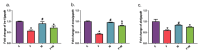




References
-
- Akar, F., Sumlu, E., Alçığır, M. E., Bostancı, A. & Sadi, G. Potential mechanistic pathways underlying intestinal and hepatic effects of kefir in high-fructose-fed rats. Food Res. Int.143, 110287 (2021). - PubMed
-
- Sumlu, E., Bostancı, A., Sadi, G., Alçığır, M. E. & Akar, F. Lactobacillus plantarum improves lipogenesis and IRS-1/AKT/eNOS signalling pathway in the liver of high-fructose-fed rats. Arch. Physiol. Biochem.128, 786–794 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

