Vitamin D receptor and its antiproliferative effect in human pulmonary arterial hypertension
- PMID: 39523384
- PMCID: PMC11551162
- DOI: 10.1038/s41598-024-78380-9
Vitamin D receptor and its antiproliferative effect in human pulmonary arterial hypertension
Abstract
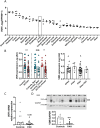
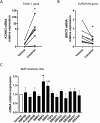
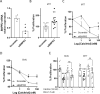
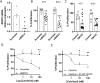
Vitamin D (vitD) deficiency is frequently observed in patients with pulmonary arterial hypertension (PAH) and, in these patients, low levels of vitD correlate with worse prognosis. The aim of this study was to examine the expression and the antiproliferative role of vitD receptor (VDR) and its signalling pathway in the human pulmonary vasculature. VDR presence and expression was analyzed in lungs, pulmonary artery smooth muscle cells (PASMC) and endothelial cells (PAEC) from controls and PAH-patients. VDR expression and VDR-target genes were examined in PASMC treated with calcitriol. The antiproliferative effect of 48 h-calcitriol was studied in PASMC by MTT and BrdU assays. VDR is expressed in PASMC. It is downregulated in lungs and in PASMC, but not in PAEC, from PAH-patients compared to non-hypertensive controls. Calcitriol strongly upregulated VDR expression in PASMC and the VDR target genes KCNK3 (encoding TASK1), BIRC5 (encoding survivin) and BMP4. Calcitriol produced an antiproliferative effect which was diminished by silencing or by pharmacological inhibition of survivin or BMPR2, but not of TASK1. In conclusion, the expression of VDR is low in PAH-patients and can be rescued by calcitriol. VDR exerts an antiproliferative effect in PASMC by modulating survivin and the BMP signalling pathway.
© 2024. The Author(s).
Conflict of interest statement
Figures


References
-
- Pullamsetti, S. S., Savai, R., Seeger, W. & Goncharova, E. A. Translational advances in the field of pulmonary hypertension. From cancer biology to new pulmonary arterial hypertension therapeutics. Targeting cell growth and proliferation signaling hubs. Am. J. Respir. Crit. Care Med.195, 425–437. 10.1164/rccm.201606-1226PP (2017). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

