Antimicrobial stewardship to reduce overtreatment of asymptomatic bacteriuria in critical access hospitals: measuring a quality improvement intervention
- PMID: 39523519
- PMCID: PMC11790331
- DOI: 10.1017/ice.2024.171
Antimicrobial stewardship to reduce overtreatment of asymptomatic bacteriuria in critical access hospitals: measuring a quality improvement intervention
Abstract
Background: Asymptomatic bacteriuria (ASB) treatment is a common form of antibiotic overuse and diagnostic error. Antibiotic stewardship using the inappropriate diagnosis of urinary tract infection (ID-UTI) measure has reduced ASB treatment in diverse hospitals. However, critical access hospitals (CAHs) have differing resources that could impede stewardship. We aimed to determine if stewardship including the ID-UTI measure could reduce ASB treatment in CAHs.
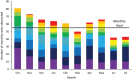
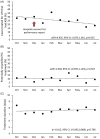
Methods: From October 2022 to July 2023, ten CAHs participated in an Intensive Quality Improvement Cohort (IQIC) program including 3 interventions to reduce ASB treatment: 1) learning labs (ie, didactics with shared learning), 2) mentoring, and 3) data-driven performance reports including hospital peer comparison based on the ID-UTI measure. To assess effectiveness of the IQIC program, change in the ID-UTI measure (ie, percentage of patients treated for a UTI who had ASB) was compared to two non-equivalent control outcomes (antibiotic duration and unjustified fluoroquinolone use).
Results: Ten CAHs abstracted a total of 608 positive urine culture cases. Over the cohort period, the percentage of patients treated for a UTI who had ASB declined (aOR per month = 0.935, 95% CI: 0.873, 1.001, P = 0.055) from 28.4% (range across hospitals, 0%-63%) in the first to 18.6% (range, 0%-33%) in the final month. In contrast, antibiotic duration and unjustified fluoroquinolone use were unchanged (P = 0.768 and 0.567, respectively).
Conclusions: The IQIC intervention, including learning labs, mentoring, and performance reports using the ID-UTI measure, was associated with a non-significant decrease in treatment of ASB, while control outcomes (duration and unjustified fluoroquinolone use) did not change.
Conflict of interest statement
All authors report no conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

