Long-term safety and tolerability of hyaluronidase-facilitated subcutaneous immunoglobulin 10% as maintenance therapy for chronic inflammatory demyelinating polyradiculoneuropathy: Results from the ADVANCE-CIDP 3 trial
- PMID: 39523874
- PMCID: PMC11625974
- DOI: 10.1111/jns.12672
Long-term safety and tolerability of hyaluronidase-facilitated subcutaneous immunoglobulin 10% as maintenance therapy for chronic inflammatory demyelinating polyradiculoneuropathy: Results from the ADVANCE-CIDP 3 trial
Abstract
Background and aims: Hyaluronidase-facilitated subcutaneous immunoglobulin (fSCIG) consists of subcutaneous human immunoglobulin G (IgG) 10% with recombinant human hyaluronidase (rHuPH20) and can be administered at the same dose and interval as intravenous IgG (IVIG). fSCIG recently received US approval as maintenance therapy for adults with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) and European approval for adults and children with CIDP after stabilization with IVIG.
Methods: ADVANCE-CIDP 3 (NCT02955355) was an open-label long-term extension of the Phase 3 double-blind randomized placebo-controlled ADVANCE-CIDP 1 study (NCT02549170) that examined fSCIG safety and efficacy as maintenance CIDP therapy. Primary outcomes were safety, tolerability, and immunogenicity. Efficacy was an exploratory outcome.
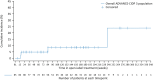
Results: The study provided 220 patient-years of follow-up data from 85 patients. Median (range) exposure was 33 (0-77) months. Patients received fSCIG every 4 weeks (88.2%) or every 3 weeks (11.8%). Median (range) 4-weekly IgG dose equivalent was 64.0 (28.0-200.0) g. Mean (standard deviation) infusion duration was 135.5 (62.8) minutes. Most adverse events (AEs) were mild or moderate and self-limiting. Of the 1406 AEs, only 48 were severe and 30 were serious. fSCIG-related AEs (n = 798) included infusion site reactions such as pain, redness, and pruritus. Three infusions (0.1%) were reduced in rate, interrupted, or stopped due to intolerability. Relapse occurred in 10 of 77 patients (13.0%); annual relapse rate was 4.5%. An anti-rHuPH20 antibody titer ≥1:160 was detected in 14 of 84 patients (16.7%); patients who tested positive (≥1:160) had similar relapse rates versus those who tested negative (16.7% vs. 12.3%, respectively).
Interpretation: ADVANCE-CIDP 3 demonstrated favorable fSCIG long-term safety and tolerability consistent with its established safety profile, and a low relapse rate, supporting use as maintenance CIDP treatment.
Keywords: ADVANCE‐CIDP 3; chronic inflammatory demyelinating polyradiculoneuropathy; hyaluronidase‐facilitated subcutaneous immunoglobulin 10%; long‐term safety and tolerability; open‐label extension study.
© 2024 The Author(s). Journal of the Peripheral Nervous System published by Wiley Periodicals LLC on behalf of Peripheral Nerve Society.
Conflict of interest statement
Figures
References
-
- Van den Bergh PY, Hadden RD, Bouche P, et al. European Federation of Neurological Societies/peripheral nerve society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the peripheral nerve society—first revision. Eur J Neurol. 2010;17:356‐363. - PubMed
-
- Van den Bergh PY, van Doorn PA, Hadden RD, et al. European academy of neurology/peripheral nerve society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force—second revision. J Peripher Nerv Syst. 2021;26:242‐268. - PubMed
-
- Boukhris S, Magy L, Gallouedec G, et al. Fatigue as the main presenting symptom of chronic inflammatory demyelinating polyradiculoneuropathy: a study of 11 cases. J Peripher Nerv Syst. 2005;10:329‐337. - PubMed
-
- Guptill JT, Bromberg MB, Zhu L, et al. Patient demographics and health plan paid costs in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2014;50:47‐51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

