Grape-Seed Proanthocyanidin Extract (GSPE) Modulates Diurnal Rhythms of Hepatic Metabolic Genes and Metabolites, and Reduces Lipid Deposition in Cafeteria-Fed Rats in a Time-of-Day-Dependent Manner
- PMID: 39523911
- PMCID: PMC11653167
- DOI: 10.1002/mnfr.202400554
Grape-Seed Proanthocyanidin Extract (GSPE) Modulates Diurnal Rhythms of Hepatic Metabolic Genes and Metabolites, and Reduces Lipid Deposition in Cafeteria-Fed Rats in a Time-of-Day-Dependent Manner
Abstract
Scope: Metabolic dysfunction-associated steatotic liver disease (MASLD) is a global health issue with increasing prevalence. Polyphenols, such as grape seed proanthocyanidin extract (GSPE), are bioactive compounds present in plants and represent an interesting therapeutical approach for MASLD.
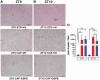
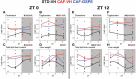
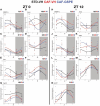
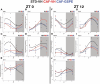
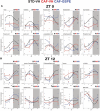
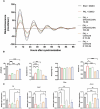
Methods and results: This study questioned whether the timing of GSPE administration impacts liver diurnal metabolism and steatosis in a rat obesity model. Results from hepatic lipid profiling and diurnal metabolic gene expression and metabolomics reveal that rats fed with a cafeteria (CAF) diet show impaired glucose homeostasis and enhanced lipogenesis in the liver, contributing to liver steatosis. Chronic consumption of GSPE in the inactive or active phase is associated with beneficial effects as the restoration of rhythms of transcripts and metabolites is observed. However, only when given in the active phase, GSPE treatment decreases hepatic triglyceride levels. Using an in vitro hepatocyte model, the study identifies that catechin, one of the main phenolic compounds found in the GSPE extract, is a potential mediator in ameliorating the effects of CAF-induced liver steatosis.
Conclusion: Taken altogether, the findings show that the beneficial effects of GSPE on MASLD development depend on the treatment time.
Keywords: NAFLD/MASLD; chronobiology; circadian rhythms; liver metabolism; obesity; proanthocyanidins.
© 2024 The Author(s). Molecular Nutrition & Food Research published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Time-of-Day Circadian Modulation of Grape-Seed Procyanidin Extract (GSPE) in Hepatic Mitochondrial Dynamics in Cafeteria-Diet-Induced Obese Rats.Nutrients. 2022 Feb 12;14(4):774. doi: 10.3390/nu14040774. Nutrients. 2022. PMID: 35215423 Free PMC article.
-
Lipogenesis is decreased by grape seed proanthocyanidins according to liver proteomics of rats fed a high fat diet.Mol Cell Proteomics. 2010 Jul;9(7):1499-513. doi: 10.1074/mcp.M000055-MCP201. Epub 2010 Mar 23. Mol Cell Proteomics. 2010. PMID: 20332082 Free PMC article.
-
Grape Seed Proanthocyanidin Extract Attenuates Cafeteria-Diet-Induced Liver Metabolic Disturbances in Rats: Influence of Photoperiod.Int J Mol Sci. 2024 Jul 14;25(14):7713. doi: 10.3390/ijms25147713. Int J Mol Sci. 2024. PMID: 39062955 Free PMC article.
-
Mechanistic insight into nuclear receptor-mediated regulation of bile acid metabolism and lipid homeostasis by grape seed procyanidin extract (GSPE).Cell Biochem Funct. 2017 Jan;35(1):12-32. doi: 10.1002/cbf.3247. Cell Biochem Funct. 2017. PMID: 28083965 Review.
-
Effects of Grape Seed Proanthocyanidin Extract on Obesity.Obes Facts. 2020;13(2):279-291. doi: 10.1159/000502235. Epub 2020 Feb 28. Obes Facts. 2020. PMID: 32114568 Free PMC article.
Cited by
-
Clinical Insights into Non-Alcoholic Fatty Liver Disease and the Therapeutic Potential of Flavonoids: An Update.Nutrients. 2025 Mar 9;17(6):956. doi: 10.3390/nu17060956. Nutrients. 2025. PMID: 40289935 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

