Mavacamten: Real-World Experience From 22 Months of the Risk Evaluation and Mitigation Strategy (REMS) Program
- PMID: 39523955
- PMCID: PMC11745710
- DOI: 10.1161/CIRCHEARTFAILURE.124.012441
Mavacamten: Real-World Experience From 22 Months of the Risk Evaluation and Mitigation Strategy (REMS) Program
Abstract
Background: Mavacamten is the only cardiac myosin inhibitor approved by the U.S. Food and Drug Administration for the treatment of patients with symptomatic New York Heart Association class II-III obstructive hypertrophic cardiomyopathy. Under the Risk Evaluation and Mitigation Strategy program for mavacamten, patients are required to be monitored for the development of systolic heart failure and reduction of left ventricular ejection fraction (LVEF) to <50%. We report results from the mavacamten Risk Evaluation and Mitigation Strategy database (April 28, 2022 to February 27, 2024).
Methods: Data on health care providers and pharmacy certification, patient monitoring (from Patient Status Forms, based partly on echocardiograms), and screening for drug interactions before each dispense were collected.
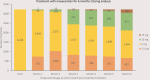
Results: Of the 6299 patients who received ≥1 dose of mavacamten, 60.0% were women; 64.6% were aged >60 years. Of the 5573 patients with submitted Patient Status Forms, 256 (4.6%) developed LVEF <50%, and 71 (1.3%) experienced heart failure requiring hospitalization. On the 29 111 status forms in these patients, each representing an assessment of an echocardiogram, LVEF <50% was reported on 276 (0.9%), and heart failure requiring hospitalization was reported on 86 (0.3%). Of the 1929 patients with ≥1 year of treatment, 78 (4.0%) had an LVEF reduction to <50%, and 4 (0.2%) experienced LVEF <50% and heart failure requiring hospitalization but later resumed treatment. Of the 3228 patients initiated on 5 mg/d mavacamten and were treated for at least 6 months, 2391 (74.1%) remained at 5 or 10 mg/d. At 3 and 6 months following mavacamten treatment initiation, 57.2% and 70.3%, respectively, demonstrated post-Valsalva left ventricular outflow tract gradient <30 mm Hg.
Conclusions: We describe the feasibility and experience of the first 22 months of the Risk Evaluation and Mitigation Strategy program for prescribing mavacamten in >6000 patients with symptomatic obstructive hypertrophic cardiomyopathy. The need for temporary interruption for LVEF <50% was low, including for patients on therapy ≥1 year, with even fewer LVEF reductions associated with heart failure requiring hospitalization.
Keywords: cardiomyopathy, hypertrophic; patient safety.
Conflict of interest statement
Dr Desai is a consultant for Bristol Myers Squibb, Cytokinetics, Viz.ai, Tenaya Therapeutics, and Edgewise Therapeutics. D. Seto, M. Cheung, Dr Afsari, N. Patel, Dr Bastien, Dr Lockman, and M. Coiro are employees of Bristol Myers Squibb. Dr Martinez is an advisory board and steering committee member as well as an unbranded speaker for Bristol Myers Squibb.
Figures



Comment in
-
Stop Dreaming: Mavacamten REMS Data Are Here.Circ Heart Fail. 2025 Jan;18(1):e012545. doi: 10.1161/CIRCHEARTFAILURE.124.012545. Epub 2024 Nov 11. Circ Heart Fail. 2025. PMID: 39523953 No abstract available.
References
-
- Maron BJ, Desai MY, Nishimura RA, Spirito P, Rakowski H, Towbin JA, Dearani JA, Rowin EJ, Maron MS, Sherrid MV. Management of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:390–414. doi: 10.1016/j.jacc.2021.11.021 - PubMed
-
- Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, Bezzina CR, Biagini E, Blom NA, de Boer RA, et al. ; ESC Scientific Document Group. 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44:3503–3626. doi: 10.1093/eurheartj/ehad194 - PubMed
-
- Ommen SR, Ho CY, Asif IM, Balaji S, Burke MA, Day SM, Dearani JA, Epps KC, Evanovich L, Ferrari VA, et al. . 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1239-e1311. doi: 10.1161/CIR.0000000000001250 - PubMed
-
- Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, et al. . Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. doi: 10.1016/j.jacc.2005.02.090 - PubMed
-
- Smedira NG, Lytle BW, Lever HM, Rajeswaran J, Krishnaswamy G, Kaple RK, Dolney DO, Blackstone EH. Current effectiveness and risks of isolated septal myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 2008;85:127–133. doi: 10.1016/j.athoracsur.2007.07.063 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

