Optimization of divalent mercury removal from synthetic wastewater using desirability function in central composite design of response surface methodology
- PMID: 39524114
- PMCID: PMC11549279
- DOI: 10.1007/s40201-023-00888-5
Optimization of divalent mercury removal from synthetic wastewater using desirability function in central composite design of response surface methodology
Abstract
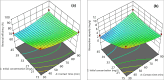
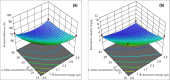
Heavy metals exist in the ecosystem both naturally and due to anthropogenic activities and as recalcitrant pollutants; they are non-biodegradable and cause acute and chronic diseases to human beings and many lifeforms. A statistical experimental approach was applied in this current study to optimize the detoxification of mercury [Hg(II)] from mono-component biosorption system by a novel hybrid granular activated carbon (biosorbent) prepared from maize plant residues. The analysis of variance by the application of central composite design shows that all the studied independent factors greatly influence Hg(II) removal efficiency and uptake capacity. The optimum experimental condition of 30 min contact time, 0.5 g/L biosorbent dosage, and 15 mg/L initial Hg(II) concentration were achieved after seeking 20 optimization solutions at 0.903 desirability. The optimum percentage removal and uptake capacity of Hg(II) at the optimal experimental setup was 96.7% and 10.8 mg/g, respectively. To confirm the quadratic models developed for the prediction of the responses as a function of the independent factors, confirmatory laboratory experiments were performed at the optimum condition. The results show that at the established best experimental condition, the optimum Hg(II) removal efficiency of 98.3% and uptake capacity of 11.2 mg/g were attained, which were within the prediction intervals indicating the suitability of the quadratic models in predicting future cases. The TEM and XRD analyses show that the Hg(II) ions were adsorbed by the biosorbent successfully and this suggests the potential and applicability of this novel biosorbent in treating water contaminants, especially heavy metals.
Supplementary information: The online version contains supplementary material available at 10.1007/s40201-023-00888-5.
Keywords: Biosorption; Mercury; Modeling; Optimization; Response surface; Wastewater.
© The Author(s), under exclusive licence to Tehran University of Medical Sciences 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Competing interestsThe authors declare no competing of interest regarding the publication of this article.
Figures






Similar articles
-
Assessment of heat-inactivated marine Aspergillus flavus as a novel biosorbent for removal of Cd(II), Hg(II), and Pb(II) from water.Environ Sci Pollut Res Int. 2017 Aug;24(22):18218-18228. doi: 10.1007/s11356-017-9323-8. Epub 2017 Jun 20. Environ Sci Pollut Res Int. 2017. PMID: 28634799
-
Experimental study and parameters optimization of microalgae based heavy metals removal process using a hybrid response surface methodology-crow search algorithm.Sci Rep. 2020 Sep 15;10(1):15068. doi: 10.1038/s41598-020-72236-8. Sci Rep. 2020. PMID: 32934284 Free PMC article.
-
Mercury(II) and lead(II) ions removal using a novel thiol-rich hydrogel adsorbent; PHPAm/Fe3O4@SiO2-SH polymer nanocomposite.Environ Sci Pollut Res Int. 2023 Jan;30(5):13605-13623. doi: 10.1007/s11356-022-23055-z. Epub 2022 Sep 22. Environ Sci Pollut Res Int. 2023. PMID: 36136188
-
Application of statistical response surface methodology for optimization of fluoride removal efficiency by Padina sp. alga.Water Environ Res. 2020 Jul;92(7):1080-1088. doi: 10.1002/wer.1305. Epub 2020 Feb 16. Water Environ Res. 2020. PMID: 32012380
-
Burkholderia tropica as a Potential Microalgal Growth-Promoting Bacterium in the Biosorption of Mercury from Aqueous Solutions.J Microbiol Biotechnol. 2017 Jun 28;27(6):1138-1149. doi: 10.4014/jmb.1611.11059. J Microbiol Biotechnol. 2017. PMID: 28301920
References
-
- Zeng H, Yu Y, Wang F, Zhang J, Li D. Arsenic(V) removal by granular adsorbents made from water treatment residuals materials and chitosan. Colloids Surf Physicochem Eng Asp. 2020;585:1–10. 10.1016/j.colsurfa.2019.124036.
-
- Lamzougui G, Es-Said A, Nafai H, Chafik D, Bouhaouss A, Bchitou R. Optimization and modeling of Pb(II) adsorption from aqueous solution onto phosphogypsum by application of response surface methodology. Phosphorus, Sulfur Silicon Relat Elem. 2021;196(6):521–9. 10.1080/10426507.2020.1860985.
-
- Kavitha V, Palanivelu K. Spectrophotometric determination of trace amounts of arsenic in sediment, flyash and water samples. Int J ChemTech Res. 2014;7(5):2333–40. https://sphinxsai.com/2015/ch_vol7_no5/3/-(2333-2340)V7N5.pdf. Accessed 28 Mar 2021.
-
- Hashemi SA, Mousavi SM, Ramakrishna S. Effective removal of mercury, arsenic and lead from aqueous media using Polyaniline-Fe3O4- silver diethyldithiocarbamate nanostructures. J Clean Prod. 2019;239:1–15. 10.1016/j.jclepro.2019.118023.
LinkOut - more resources
Full Text Sources

