Tert-butyl hydroperoxide induces trabecular meshwork cells injury through ferroptotic cell death
- PMID: 39524143
- PMCID: PMC11544637
- DOI: 10.1002/ccs3.12050
Tert-butyl hydroperoxide induces trabecular meshwork cells injury through ferroptotic cell death
Abstract
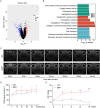
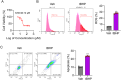
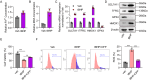
Trabecular meshwork (TM) tissue has a crucial role in regulating aqueous humor circulation in the eye, thus maintaining normal intraocular pressure (IOP). TM dysfunction causes IOP elevation, which leads to glaucoma. To investigate biological changes in TM tissue in patients with glaucoma, we analyzed the mRNA expression microarray dataset, GSE27276. Gene ontology analysis indicated that redox microenvironment imbalance is among the main changes of TM tissue in patients with glaucoma. Subsequently, we induced oxidative stress in TM cells using the tert-butyl hydroperoxide (tBHP) treatment, to generate in vivo and in vitro models, and conducted mRNA sequencing to identify genes with critical roles in maintaining the redox microenvironment balance. We found that the tBHP caused TM dysfunction in vivo, characterized by aqueous humor circulation resistance, IOP elevation, and TM cell death. Further, Kyoto Encyclopedia of Genes and Genomes pathway analysis showed that ferroptosis signaling was enriched in tBHP-treated TM cells. Consistently, in vitro analyses showed that levels of reactive oxygen species, ferric ion, and malondialdehyde were increased after the tBHP treatment, indicating TM cell ferroptosis. Furthermore, inhibiting ferroptosis alleviated tBHP-induced TM cell injury. This study provides new insights suggesting that inhibition of ferroptosis has potential as a treatment for glaucoma.
Keywords: ferroptosis; glaucoma; oxidative stress; trabecular meshwork.
© 2024 The Author(s). Journal of Cell Communication and Signaling published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Ion channel Piezo1 induces ferroptosis of trabecular meshwork cells: a novel observation in the pathogenesis in primary open angle glaucoma.Am J Physiol Cell Physiol. 2024 Dec 1;327(6):C1591-C1603. doi: 10.1152/ajpcell.00173.2024. Epub 2024 Oct 28. Am J Physiol Cell Physiol. 2024. PMID: 39466179 Free PMC article.
-
Metformin protects trabecular meshwork against oxidative injury via activating integrin/ROCK signals.Elife. 2023 Jan 4;12:e81198. doi: 10.7554/eLife.81198. Elife. 2023. PMID: 36598818 Free PMC article.
-
Activation of ATF4 triggers trabecular meshwork cell dysfunction and apoptosis in POAG.Aging (Albany NY). 2021 Mar 10;13(6):8628-8642. doi: 10.18632/aging.202677. Epub 2021 Mar 10. Aging (Albany NY). 2021. PMID: 33714955 Free PMC article.
-
Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: challenges of dual combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of nonhydrolyzed carnosine.Fundam Clin Pharmacol. 2012 Feb;26(1):86-117. doi: 10.1111/j.1472-8206.2011.00969.x. Epub 2011 Aug 24. Fundam Clin Pharmacol. 2012. PMID: 21883446 Review.
-
Cell-Based Therapies for Trabecular Meshwork Regeneration to Treat Glaucoma.Biomolecules. 2021 Aug 24;11(9):1258. doi: 10.3390/biom11091258. Biomolecules. 2021. PMID: 34572471 Free PMC article. Review.
References
-
- Amankwa, C. E. , Young O., DebNath B., Gondi S. R., Rangan R., Ellis D. Z., Zode G., Stankowska D. L., and Acharya S.. 2023. “Modulation of Mitochondrial Metabolic Parameters and Antioxidant Enzymes in Healthy and Glaucomatous Trabecular Meshwork Cells with Hybrid Small Molecule SA‐2.” International Journal of Molecular Sciences 24(14): 11557. 10.3390/ijms241411557. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

