Heterogeneity in adverse events related to atezolizumab-bevacizumab for hepatocellular carcinoma reported in real-world studies
- PMID: 39524204
- PMCID: PMC11550199
- DOI: 10.1016/j.jhepr.2024.101190
Heterogeneity in adverse events related to atezolizumab-bevacizumab for hepatocellular carcinoma reported in real-world studies
Abstract
Background & aims: Safety data for patients with hepatocellular carcinoma (HCC) treated with atezolizumab-bevacizumab in the real-world setting remain uncertain. Thus, the aim of this study was to evaluate the incidence of adverse events (AEs) in patients with HCC treated with atezolizumab-bevacizumab in the literature.
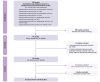
Methods: In this systematic review and meta-analysis, we searched PubMed for original studies reporting percentages of AEs in patients with HCC receiving atezolizumab-bevacizumab between 2020 to 2023, using the search terms "Atezolizumab/Bevacizumab", "HCC" and "Adverse events". We summarized the incidence of AEs and performed a meta-analysis in order to evaluate the incidence of AEs reported in the literature.
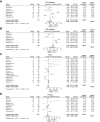
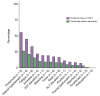
Results: A total of 30 studies (3,867 patients) were included. The analysis revealed heterogeneity in AE reporting, with arterial hypertension, proteinuria, and fatigue being the most frequently reported AEs whereas incidence of bleeding was reported in 66.7% of the studies and rare immune-related AEs were reported in 26.7% of the studies. The meta-analysis revealed pooled incidence rates of 79% for any grade AEs: 56% for grade 1/2 and 30% for grade ≥3. While the pooled rates of hypertension, anorexia, bleeding, pruritus, rash, and thyroid dysfunction were similar to those reported in the IMbrave150 trial, higher rates were observed in the literature for proteinuria, fatigue, ALT and AST elevations and gastrointestinal perforation. For grade ≥3 AEs, the percentages were consistent with the IMbrave150 trial, except for lower incidences of arterial hypertension and thrombosis in the literature. The exposure-adjusted incidence rates for proteinuria (55.7%), hypertension (45.3%) and fatigue (33.6%) were high. Heterogeneity was observed in the analysis of AEs across articles within the same cohorts of patients.
Conclusion: We observed a significant variability in AE reporting for atezolizumab-bevacizumab treatment in HCC in the literature, underscoring the need for standardized reporting practices.
Impact and implications: Considering the demonstrated safety of atezolizumab-bevacizumab in randomized-controlled trials, this meta-analysis offers valuable insights into reported occurrences of adverse events. Our study highlights significant heterogeneity among studies, underscoring the need to improve adverse event recording. Understanding the incidence and severity of treatment-related adverse events beyond clinical trials is essential for prompt intervention and may help in preventing treatment discontinuation and complications, potentially leading to better outcomes without significantly compromising quality of life due to adverse events.
Keywords: adverse effects; immunotherapy; liver cancer.
© 2024 The Author(s).
Figures




References
-
- Llovet J.M., Kelley R.K., Villanueva A., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021 Dec;7(1):6. - PubMed
-
- Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020 May 14;382(20):1894–1905. - PubMed
-
- Cheng A.L., Qin S., Ikeda M., et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022 Apr;76(4):862–873. - PubMed
LinkOut - more resources
Full Text Sources

