Liver stiffness measurement predicts clinical outcomes in autoimmune hepatitis
- PMID: 39524208
- PMCID: PMC11550196
- DOI: 10.1016/j.jhepr.2024.101213
Liver stiffness measurement predicts clinical outcomes in autoimmune hepatitis
Abstract
Background & aims: Liver stiffness measurement (LSM) has been shown to adequately predict outcomes in patients with liver disease. However, the value of LSM as a predictor of disease progression in autoimmune hepatitis (AIH) remains to be determined. This study aimed to evaluate the role of LSM as a predictor of disease progression and decompensation of cirrhosis in patients with AIH.
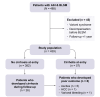
Methods: This multicentre cohort study included 439 patients with histologically confirmed AIH and at least one LSM during follow-up. The association between the first LSM performed at least 6 months after treatment initiation (baseline LSM [BLSM]) and cirrhosis development and poor outcomes (decompensation, liver transplantation, and/or liver-related death) was assessed using Cox regression and its discriminating capacity with a receiver-operating characteristic curve.
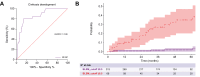
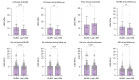
Results: Most patients were female (n = 301, 70%), with a median age of 52 years. BLSM performed after a median of 2.18 (1.19-4.68) years had a median value of 6 kPa (4.5-8.5). At the time of BLSM, 332 (76%) patients had achieved a biochemical response and 57 (13%) had cirrhosis. During follow-up, eight patients (2%) presented with poor outcomes and 26 (7%) developed cirrhosis. BLSM was higher among patients with poor outcomes (13.5 kPa vs. 6 kPa; p <0.001) and was independently associated with cirrhosis development (hazard ratio 1.300; p <0.001), irrespective of the achievement of biochemical response. A cut-off of 8.5 kPa accurately predicted cirrhosis development and poor outcomes, with AUCs of 0.859 (95% CI 0.789-0.929) and 0.900 (95% CI 0.847-0.954), respectively.
Conclusion: BLSM could play a significant role in predicting AIH outcomes, potentially identifying a subgroup of patients at a high risk of progressing to cirrhosis and experiencing decompensation.
Impact and implications: The value of liver stiffness measurement as a predictor of outcomes in patients with autoimmune hepatitis (AIH) remains to be determined. In this large multicentre study, liver stiffness measurement was found to be an independent predictive factor of adverse clinical outcomes and cirrhosis development in AIH, irrespective of the achievement of biochemical response. A cut-off of 8.5 kPa accurately predicted cirrhosis development and poor outcomes in AIH.
Keywords: autoimmune hepatitis; cirrhosis; decompensation; elastography; liver stiffness measurement; outcome.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
LinkOut - more resources
Full Text Sources

